Largest genetic study of mosquitoes reveals spread of insecticide resistance across Africa
Malaria is transmitted by mosquitoes and rising resistance to insecticides is hampering efforts to control the disease. The study by researchers from the Wellcome Trust Sanger Institute and their collaborators also discovered that wild mosquitoes collected in Africa were genetically far more diverse than had been thought. This helps to explain how mosquitoes evolve insecticide resistance so quickly.
More than 200 million people are infected with the malaria parasite worldwide each year, which is transmitted by blood-sucking Anopheles mosquitoes. Malaria caused the deaths of around 429,000 people in 2015* with the majority of cases in sub-Saharan Africa.
Public health measures in Africa such as insecticide-treated bed nets and insecticide-spraying have helped reduce the numbers of malaria cases since 2000, but many mosquitoes have evolved resistance to insecticides. This is now threatening to derail malaria control in Africa.
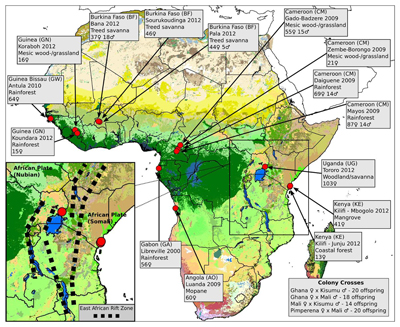
To understand how mosquitoes are evolving, researchers working with the Anopheles gambiae 1000 genomes project sequenced the DNA of 765 wild Anopheles mosquitoes. These were taken from 15 locations across eight African countries, creating the largest data resource on natural genetic variation for any species of insect. They then examined each of the mosquito genomes.
The researchers revealed that the Anopheles gambie mosquitoes are extremely genetically diverse compared with most other animal species. High genetic diversity enables rapid evolution and the study found 52 million small differences amongst the mosquito genomes.
“The diversity of mosquito genomes was far greater than we expected. Such high levels of genetic variation poise mosquito populations to rapidly evolve in response to our efforts to control them whether that be with insecticides or any other control measure, including gene drive.”
Dr Mara Lawniczak A corresponding author on the paper and Faculty at the Wellcome Trust Sanger Institute
New strategies to control mosquitoes are being developed that use ‘gene drive’– using the latest Crispr/Cas 9 genetic tools to make mosquitoes infertile or unable to carry the malaria parasite. However, this technology requires an exact match with any targeted gene. The researchers found that gene drive is unlikely to work for most mosquito genes because they are too variable in nature, however they also used the data to highlight less variable targets that are potentially more suitable for gene drive based methods to control mosquitoes.
The mosquito genomes also revealed rapid evolution of several genes that had previously been implicated in insecticide resistance. Unexpectedly, the researchers discovered many previously-unknown genetic variants within those genes that could be causing insecticide resistance. Worryingly, they showed that these genetic variants for insecticide resistance were not only emerging independently in different parts of Africa, but were also being spread across the continent by mosquito migration.
“We know that mosquito populations are rapidly evolving resistance to insecticides, which is a serious threat to the future of malaria control in Africa. We have been able to see that a diverse array of genes linked to insecticide resistance are under very strong selection, confirming that they are playing an important role in the evolution of insecticide resistance in natural mosquito populations. Our study highlights the severe challenges facing public efforts to control mosquitoes and to manage and limit insecticide resistance.”
Professor Martin Donnelly A corresponding author from the Liverpool School of Tropical Medicine and Honorary Faculty at the Wellcome Trust Sanger Institute
“The data we have generated are a unique resource for studying how mosquito populations are responding to our current control efforts, and for designing better technologies and strategies for mosquito control in the future. More data will be needed to fill in the geographical gaps and study how mosquito populations change over time and in response to specific control interventions. However, this study demonstrates a clear path towards building a new and much-needed source of intelligence to support the campaign to eradicate malaria in Africa.”
Alistair Miles Lead author from the University of Oxford and the Wellcome Trust Sanger Institute
More information
*WHO stats on malaria http://www.who.int/features/factfiles/malaria/en/
For more information about malaria please see https://www.yourgenome.org/facts/what-is-malaria
Funding:
This work was supported by the Wellcome Trust, Medical Research Council UK, the Department for International Development, the Foundation for the National Institutes of Health through the Vector-Based Control of Transmission: Discovery Research (VCTR) program of the Grand Challenges in Global Health initiative of the Bill & Melinda Gates Foundation and the National Institute of Allergy and Infectious Diseases (NIAID).
Participating Centres:
- Malaria Programme, Wellcome Trust Sanger Institute, Hinxton, Cambridge CB10 1SA, UK.
- MRC Centre for Genomics and Global Health, University of Oxford, Oxford OX3 7BN, UK.
- Instituto Pasteur Italia – Fondazione Cenci Bolognetti, Dipartimento di Sanita Pubblica e Malattie Infettive, Università di Roma SAPIENZA, Rome, Italy.
- Department of Vector Biology,Liverpool School of Tropical Medicine, Pembroke Place, Liverpool L3 5QA, UK.
- University of Montana, Missoula, Montana 59812, USA.
- Department of Genetics, Rutgers University, 604 Alison Road, Piscataway, New Jersey 08854, USA.
- Genome Sequencing and Analysis Program, Broad Institute, 415 Main Street, Cambridge, Maryland 02142, USA.
- Department of Entomology, Virginia Tech, Blacksburg, Virginia 24061, USA.
- Laboratory of Ecology, Genetics and Environmental Protection, Tomsk State University, Tomsk 634050, Russia.
- Eck Institute for Global Health, Department of Biological Sciences, University of Notre Dame, Indiana 46556, USA.
- Groningen Institute for Evolutionary Life Sciences (GELIFES), Nijenborgh 7, 9747 AG Groningen, The Netherlands.
- Unité d’Ecologie des Systèmes Vectoriels, Centre International de Recherches Médicales de Franceville, Franceville, Gabon.
- Institut de Recherche pour le Développement (IRD), UMR MIVEGEC (UM1, UM2, CNRS 5290, IRD 224), Montpellier, France.
- Department of Life Sciences, Imperial College, Silwood Park, Ascot, Berkshire SL5 7PY, UK.
- Department of Zoology, University of Oxford, The Tinbergen Building, South Parks Road, Oxford OX1 3PS, UK.
- Department of Biology and School of Informatics and Computing, Indiana University, Bloomington, Indiana 47405, USA.
- KEMRI-Wellcome Trust Research Programme, PO Box 230, Bofa Road, Kilifi, Kenya. 18Global Health and Tropical Medicine, GHTM, Instituto de Higiene e Medicina Tropical, IHMT, Universidade Nova de Lisboa, UNL, Rua da Junqueira 100, 1349-008 Lisbon, Portugal.
- Department of Microbiology and Immunology, Microbial and Plant Genomics Institute, University of Minnesota, St Paul, Minnesota 55108, USA.
- Unit for Genetics and Genomics of Insect Vectors, Institut Pasteur, Paris, France.
- School of Natural Sciences and Psychology, Liverpool John Moores University, Liverpool L3 3AF, UK.
- Department of Entomology, University of California, Riverside, California, USA.
- Programa Nacional de Controle da Malária, Direcção Nacional de Saúde Pública, Ministério da Saúde, Luanda, Angola.
- Institut de Recherche en Sciences de la Santé (IRSS), Bobo Dioulasso, Burkina Faso.
- Laboratoire de Recherche sur le Paludisme, Organisation de Coordination pour la lutte contre les Endémies en Afrique Centrale (OCEAC),Yaoundé, Cameroon.
- Malaria Research and Training Centre (MRTC), University of Bamako, Mali.
- Instituto Nacional de Saúde Pública, Ministério da Saúde Pública, Bissau, Guiné-Bissau.
- Infectious Diseases Research Collaboration, 2C Nakasero Hill Road, PO Box 7475, Kampala, Uganda.
- The Broad Institute of Massachusetts Institute of Technology and Harvard, 415 Main Street, Cambridge, Massachusetts 02142, USA.
Publications:
Selected websites
About the Ag1000G project
The Anopheles gambiae 1000 genomes project (Ag1000G) was established in 2014 to sequence the genomes of mosquitoes collected from natural populations across Africa. Led by the Wellcome Trust Sanger Institute, the Ag1000G project is supported by an international consortium of 20 research institutions. The primary goal of the Ag1000G project is to generate data on natural genetic variation within and between mosquito populations in different parts of Africa. Data on genetic variation are a fundamental tool for many fields of biological research, including basic research in fundamental areas such as evolution and ecology, and applied research into new tools for mosquito control. https://www.malariagen.net/projects/ag1000g
Liverpool School of Tropical Medicine (LSTM)
Liverpool School of Tropical Medicine (LSTM) is the world’s oldest centre of excellence in tropical medicine and international public health. It has been engaged in the fight against infectious, debilitating and disabling diseases since 1898 and continues to break the cycle of poor health and poverty with a research portfolio in excess of well over £210 million and a teaching programme attracting students from over 65 countries. For further information please visit www.lstmed.ac.uk
Big Data Institute, University of Oxford
The Big Data Institute is located in the Li Ka Shing Centre for Health Informatics and Discovery at the University of Oxford. It is an interdisciplinary research centre that focuses on the analysis of large, complex data sets for research into the causes, consequences, prevention and treatment of disease. Research is conducted in areas such as genomics, population health, infectious disease surveillance and the development of new analytic methods. The Big Data Institute is supported by funding from the Medical Research Council, the UK Research Partnership Investment Fund, the National Institute for Health Research Oxford Biomedical Research Centre, and philanthropic donations from the Li Ka Shing and Robertson Foundations. Further details are available at www.bdi.ox.ac.uk
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute is one of the world’s leading genome centres. Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease. To celebrate its 25th year in 2018, the Institute is sequencing 25 new genomes of species in the UK. Find out more at www.sanger.ac.uk or follow @sangerinstitute
Wellcome
Wellcome exists to improve health for everyone by helping great ideas to thrive. We’re a global charitable foundation, both politically and financially independent. We support scientists and researchers, take on big problems, fuel imaginations and spark debate. wellcome.org


