Mutant cells colonise our tissues over our lifetime
By the time we reach middle age, more than half of the oesophagus in healthy people has been taken over by cells carrying mutations in cancer genes, scientists have uncovered. By studying normal oesophagus tissue, scientists at the Wellcome Sanger Institute, MRC Cancer Unit, University of Cambridge and their collaborators uncovered a hidden world of mutations and evolution in our tissues as we age.
The results, published today (18 October) in Science show how mutant cells mutate and compete with each other throughout life, and only the fittest mutations survive.
Every person accumulates genetic changes, or mutations, throughout their lifetime. These mutations in normal tissue, called somatic mutations, are key to understanding the first steps to cancer and likely contribute towards ageing, but are unchartered territory due to technical limitations.
For the first time, scientists have uncovered that on average, healthy cells in the oesophagus carry at least several hundred mutations per cell in people in their twenties, rising to over 2,000 mutations per cell later in life. Only mutations in a dozen or so genes seem to matter however, as these give the cells a competitive advantage allowing them to take over the tissue and form a dense patchwork of mutations.
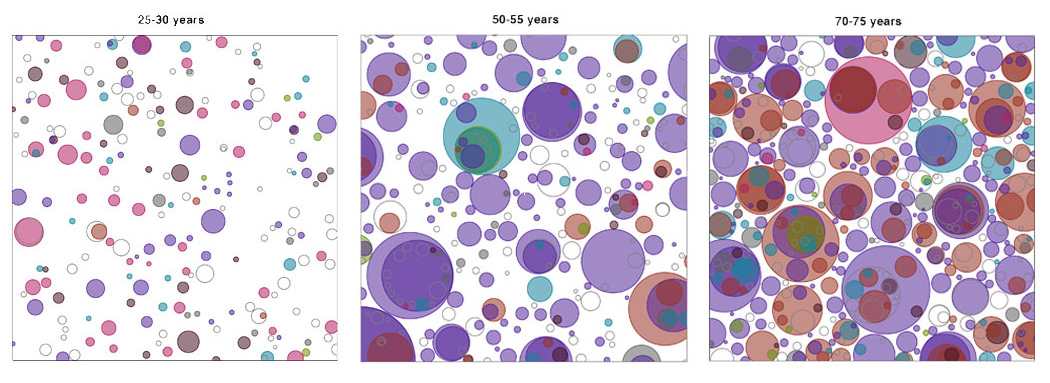
“Under the microscope, the oesophageal tissue looked completely normal – it came from healthy individuals who had no signs of cancer. After studying the genetics we were shocked to see that the healthy oesophagus was riddled with mutations. We discovered that by the time an individual reaches middle age, they probably have more mutant than normal cells.”
Professor Phil Jones Joint lead author from the Wellcome Sanger Institute and MRC Cancer Unit, University of Cambridge
The team used targeted and whole-genome sequencing to map groups of mutant cells in normal oesophageal tissue from nine individuals aged 20 to 75 years*. The individuals’ oesophageal tissues were considered healthy as none of the donors had a known history of oesophageal cancer, nor were taking medication for problems relating to the oesophagus.
The study also casts new light on the mutations that are found in the squamous kind of oesophageal cancers. One mutated gene, TP53, which is found in almost all oesophageal cancers is already mutated in 5-10 per cent of normal cells, suggesting that cancer develops from this minority of cells.
In contrast, mutations in the NOTCH1 gene, known to control cell division, were found in nearly half of all cells of normal oesophagus by middle age, being several times more common in normal tissue than cancer. This observation suggests that researchers need to reconsider the role of some genes recurrently mutated in cancer in the light of mutations in normal tissue, and raises the possibility that the NOTCH1 mutation may even protect cells against cancer development.
“For years we have sequenced cancer genomes and looked for genes that are commonly mutated across patients. We assumed that the common mutations are the ones driving the cancer. However, now we have looked at normal tissues we were surprised to find that a gene commonly associated with oesophageal cancer, NOTCH1, was more mutated in normal cells than cancer cells. These results suggest that scientists may need to rethink the role of some cancer genes in the light of sequencing normal tissues.”
Dr Jo Fowler Joint first author from the Wellcome Sanger Institute
The discovery that normal aged oesophagus is a dense patchwork of mutant cells carrying mutations previously linked with oesophageal cancer has important implications. It provides insights into key genes that control cell behaviour in normal tissues. It also gives a window into the first steps in the development of some oesophageal cancers, which are believed to arise from these mutant cells, and will be informative for current research efforts on early detection of cancer.
“We have found that genetic mutations associated with cancer are widespread in normal tissues, revealing how our own cells mutate, compete and evolve to colonise our tissues as we age. Given the importance of these mutations to cancer, it is remarkable that we have been unaware of the extent of this phenomenon until now. While the work sheds light on early cancer development, it also raises many questions about how these mutations may contribute to ageing and other diseases, opening interesting avenues for future research.”
Dr Inigo Martincorena Joint lead author from the Wellcome Sanger Institute
“As cancer researchers, we can’t underestimate the importance of studying healthy tissue. Our risk of developing cancer increases as we age, and this research brings us closer to uncovering clues within our normal tissues to help us identify individuals at higher risk of the disease.
“This study shows that some genetic changes linked to cancer are present in surprisingly large numbers of normal cells. We still have a long way to go to fully understand the implications of these new findings, but we hope that studies like this will one day help us to develop targeted diagnostic tests. In particular, oesophageal cancer is very hard to treat so detecting signs of the disease at the earliest possible stage could make a huge difference for patients.”
Professor Karen Vousden Chief scientist at Cancer Research UK, which part-funded the study
More information
*The researchers are very grateful to the families of deceased donors for their consent, and thank the Cambridge Biorepository for Translational Medicine for access to human tissue. The researchers would also like to acknowledge the work of Dr Kourosh Saeb-Parsy who swiftly transported the donors’ organs, enabling access to healthy tissue for this research.
Publication:
Inigo Martincorena, Jo Fowler et al. (2018) Somatic mutant clones colonize the human esophagus with age. Science. DOI: 10.1126/science.aau3879
Funding:
This research was supported by Cancer Research UK (C609/A17257), the Medical Research Council and Wellcome.
About Cancer Research UK
- Cancer Research UK is the world’s leading cancer charity dedicated to saving lives through research
- Cancer Research UK’s pioneering work into the prevention, diagnosis and treatment of cancer has helped save millions of lives.
- Cancer Research UK receives no funding from the UK government for its life-saving research. Every step it makes towards beating cancer relies on vital donations from the public.
- Cancer Research UK has been at the heart of the progress that has already seen survival in the UK double in the last 40 years.
- Today, 2 in 4 people survive their cancer for at least 10 years. Cancer Research UK’s ambition is to accelerate progress so that by 2034, 3 in 4 people will survive their cancer for at least 10 years.
- Cancer Research UK supports research into all aspects of cancer through the work of over 4,000 scientists, doctors and nurses.
- Together with its partners and supporters, Cancer Research UK’s vision is to bring forward the day when all cancers are cured.
For further information about Cancer Research UK’s work or to find out how to support the charity, please call 0300 123 1022 or visit www.cancerresearchuk.org. Follow us on Twitter and Facebook.
Selected websites
About the Medical Research Council Cancer Unit:
The Medical Research Council (MRC) Cancer Unit undertakes world-leading research into the earliest steps in cancer development that can be translated into clinical practice to improve the diagnosis and treatment of cancers. Its research programmes encompass a range of areas including the BRCA2-related forms of inherited cancers and the role of genomic instability in cancer progression, linkages between cancer and metabolism, cancer stem cells, the role of the tumour microenvironment in cancer development and cancer of the oesophagus. The Unit is based on the Cambridge Biomedical Campus, and is part of the University of Cambridge. www.mrc-cu.cam.ac.uk
About the Medical Research Council:
The Medical Research Council has been at the forefront of scientific discovery to improve human health. Founded in 1913 to tackle tuberculosis, the MRC now invests taxpayers’ money in some of the best medical research in the world across every area of health. Twenty-two MRC-funded researchers have won Nobel prizes in a wide range of disciplines, and MRC scientists have been behind such diverse discoveries as vitamins, the structure of DNA and the link between smoking and cancer, as well as achievements such as pioneering the use of randomised controlled trials, the invention of MRI scanning, and the development of a group of antibodies used in the making of some of the most successful drugs ever developed. Today, MRC-funded scientists tackle some of the greatest health problems facing humanity in the 21st century, from the rising tide of chronic diseases associated with ageing to the threats posed by rapidly mutating micro-organisms. www.mrc.ac.uk
About the University of Cambridge:
The mission of the University of Cambridge is to contribute to society through the pursuit of education, learning and research at the highest international levels of excellence. To date, 98 affiliates of the University have won the Nobel Prize. Founded in 1209, the University comprises 31 autonomous Colleges, which admit undergraduates and provide small-group tuition, and 150 departments, faculties and institutions. Cambridge is a global university. Its 19,000 student body includes 3,700 international students from 120 countries. Cambridge researchers collaborate with colleagues worldwide, and the University has established larger-scale partnerships in Asia, Africa and America. The University sits at the heart of the ‘Cambridge cluster’, which employs 60,000 people and has in excess of £12 billion in turnover generated annually by the 4,700 knowledge-intensive firms in and around the city. The city publishes 341 patents per 100,000 residents. www.cam.ac.uk
The Wellcome Sanger Institute
The Wellcome Sanger Institute is one of the world’s leading genome centres. Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease. To celebrate its 25th year in 2018, the Institute is sequencing 25 new genomes of species in the UK. Find out more at www.sanger.ac.uk or follow @sangerinstitute
Wellcome
Wellcome exists to improve health for everyone by helping great ideas to thrive. We’re a global charitable foundation, both politically and financially independent. We support scientists and researchers, take on big problems, fuel imaginations and spark debate. wellcome.org


