Journey to precision cancer treatment takes off with new passports tool
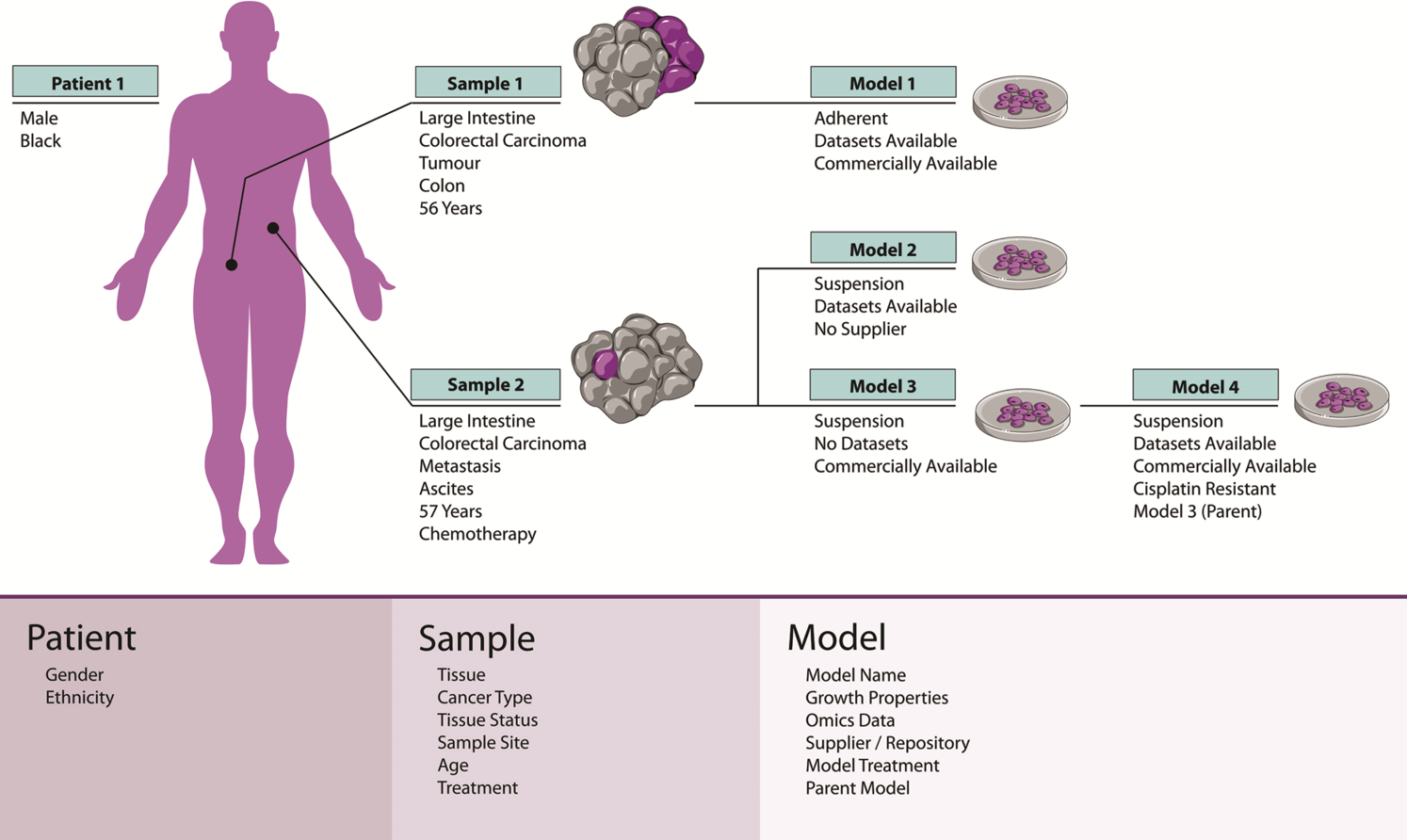
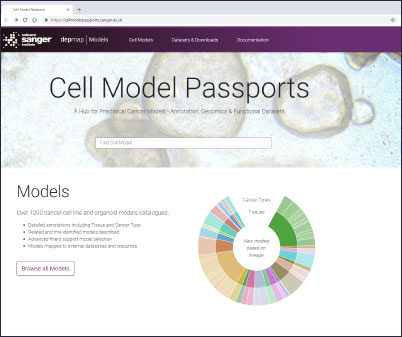
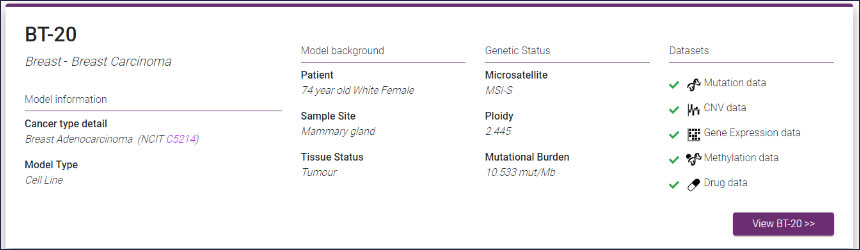
Cell Model Passports, a user-friendly website described in Nucleic Acids Research will enable cancer researchers in both academia and industry not only to access high-quality raw and processed genomic and functional datasets but also to select the best model(s) for their research. Before now, finding the most relevant cancer model(s) has often been difficult and time consuming – the Passports will streamline this process.
“We are offering a ‘one-stop-shop’ for cancer researchers. With Cell Model Passports we have combined quality controlled data on the genetics, clinical history and drug sensitivities for different cancer types to form a single, user-friendly hub for the first time. By freely sharing this data we aim to provide the shortcuts that will enable researchers around the world to accelerate cancer research.”
Syd Barthorpe Joint first author at the Wellcome Sanger Institute and co-curator of Cell Model Passports
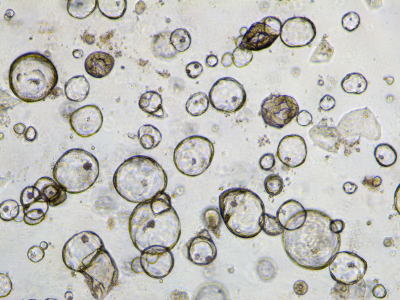
The organoids that form part of the Cell Model Passports are grown from fresh tumour tissues that are sent to the Sanger Institute from four clinical sites across the UK and are part of the Human Cancer Models Initiative, an international project to generate new cancer cell models.
The Cell Model Passports hub paves the way for the Cancer Dependency Map, or Cancer DepMap – a rulebook for the precision treatment of cancer. Through the use of organoid technology, genome sequencing, gene knock-out experiments and drug testing, scientists are identifying the weak spots of different cancers. As a result of this work, new guidelines for the future of precision cancer treatments will be created and shared.
“With organoid technology we are able to grow tumours in a dish and gain new insights into how cancers develop and respond to different drugs. Next on our agenda is to produce more cell models for cancers of high clinical unmet need. We believe the Cell Model Passports will streamline cancer research and will be a critical foundation for a cancer DepMap.”
Dr Hayley Francies Wellcome Sanger Institute’s co-lead author and co-curator of Cell Model Passports
The Cancer Dependency Map is an international effort, with the Broad Institute in the United States, to bridge the translational gap that exists between genomic sequencing and providing precision medicine to the many cancer patients. Currently, scientists do not fully understand the consequences of genetic alterations that occur in cancer. What is known is that when an error impacts a critical gene, a cancerous cell will adapt by adjusting other genes’ activity. These adaptations represent dependencies: vulnerabilities that might serve as targets for designing new therapies or repurposing existing ones. Mapping these dependencies is essential to making precision cancer medicine a reality.
“In 10 years’ time we aspire to provide precision medicine for the majority of cancer patients. The Cancer DepMap – a rulebook for selectively targeting cancer cells – will empower a new generation of targeted treatment for patients.”
Dr Mathew Garnett Leader of the Cancer Dependency Map project at the Wellcome Sanger Institute
More information
Funding:
This research was supported by CRUK (C44943/A22536), SU2C (SU2C-AACRDT1213), Wellcome (102696) and Wellcome Sanger Institute core funding (206194).
Publications:
Selected websites
Wellcome Sanger Institute
The Wellcome Sanger Institute is one of the world’s leading genome centres. Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease. To celebrate its 25th year in 2018, the Institute is sequencing 25 new genomes of species in the UK. Find out more at www.sanger.ac.uk or follow @sangerinstitute on Twitter, Facebook and LinkedIn.
Wellcome
Wellcome exists to improve health for everyone by helping great ideas to thrive. We’re a global charitable foundation, both politically and financially independent. We support scientists and researchers, take on big problems, fuel imaginations and spark debate. wellcome.org


