Salivary gland cells revealed as sites of COVID-19 infection
Scientists have shown that SARS-CoV-2, the virus that causes COVID-19, can infect specific cells in the salivary gland in the mouth. The study by researchers from the Wellcome Sanger Institute, National Institutes of Health and the University of North Carolina at Chapel Hill, and their collaborators within the Human Cell Atlas Oral & Craniofacial Network, also discovered that live cells from the mouth were found in saliva, and that the virus was able to reproduce within these infected cells.
The study revealed that salivary gland cells could play a role in transmission of the SARS-CoV-2 virus to the lungs or digestive system via saliva. Understanding the involvement of mouth cells could inform strategies to reduce viral transmission within and outside the body.
Reported today (25 March) in Nature Medicine, this first publication with the HCA Oral & Craniofacial Network is part of the international Human Cell Atlas (HCA) consortium effort to map every cell type in the human body, transforming our understanding of health, infection and disease. The study could also help explain some of the oral symptoms experienced by COVID-19 patients, including taste loss, dry mouth and blistering.
Previous studies* have shown that cells in the nose and lung contain high levels of RNA for key proteins that allow the SARS-CoV-2 virus to enter cells. However, the role of the mouth in COVID-19 transmission is poorly understood. While it is known that saliva of people with COVID-19 can contain SARS-CoV-2, it has been unclear if mouth cells are involved.
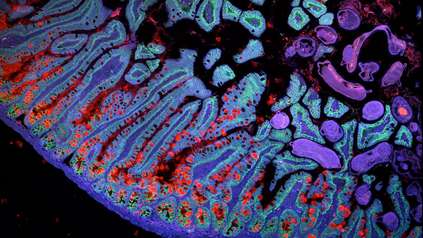
To investigate the role of mouth cells, the researchers first studied mouth tissue samples from healthy volunteers, using cutting-edge single cell RNA sequencing technology and bioinformatics methods. They looked for individual cells that expressed two key entry proteins – ACE2 and the TMPRSS2 protease – which SARS-CoV-2 uses to infect human cells, and discovered that salivary gland ductal cells and some gingival, or gum, cells expressed both proteins. This showed that these cells were vulnerable to infection.
Next, the researchers investigated mouth tissues from COVID-19 patients who had died or who had given biopsy samples. They discovered SARS-CoV-2 RNA in salivary gland cells, indicating these cells had been infected and found evidence that the virus was replicating in some of these cells.
The study also discovered that saliva from people with mild or asymptomatic COVID-19 contained mouth cells carrying SARS-CoV-2 RNA and RNA for the entry proteins. When saliva from eight of the asymptomatic people was added to monkey cells grown in dishes, some of these cells became infected. This raises the possibility that even people without symptoms might transmit infectious SARS-CoV-2 to others through saliva.
“Taken together, the study’s findings suggest that the mouth, via infected oral cells, plays a bigger role in SARS-CoV-2 infection than previously thought. When infected saliva is swallowed or tiny particles of it are inhaled, we think it can potentially transmit SARS-CoV-2 further into our throats, our lungs, or even our guts.”
Professor Kevin M. Byrd, joint lead author on the study and a coordinator of the HCA Oral & Craniofacial Biological Network, who carried out the work at the Adams School of Dentistry at the University of North Carolina at Chapel Hill
Finally, to explore the relationship between oral symptoms and virus in saliva, the team collected saliva from a separate group of 35 NIH volunteers with mild or asymptomatic COVID-19. Of the 27 people who experienced symptoms, those with virus in their saliva were more likely to report loss of taste and smell, suggesting that oral infection might underlie oral symptoms of COVID-19.
“By revealing a potentially underappreciated role for the oral cavity in SARS-CoV-2 infection, our study could open up new investigative avenues leading to a better understanding of the course of infection and disease. Such information could also inform interventions to combat the virus and alleviate oral symptoms of COVID-19.”
Professor Blake M. Warner, assistant clinical investigator and chief of NIDCR’s Salivary Disorders Unit, who co-led the study
The work was carried out as part of the global Human Cell Atlas consortium** which aims to create reference maps of all human cells to understand health and disease. More than 2,000 people across 75 countries are involved in the HCA community, and the data is openly available to scientists worldwide.
The rapid collaboration between researchers on this study led to the creation of the HCA Oral and Craniofacial Biological Network. This is working towards the goal of creating comprehensive maps of oral and craniofacial cells as a basis for understanding oral health and oral diseases.
“Human Cell Atlas data is being used to understand COVID-19 and identify which of our cells are critical for initial infection and transmission. This first integrated adult Human Oral Cell Atlas is openly available, to help understand SARS-Cov-2 transmission and inform preventative measures to reduce the spread of this coronavirus. The global Human Cell Atlas community will continue to investigate cells and targets likely to be involved in COVID-19.”
Dr Sarah Teichmann, a senior author from the Wellcome Sanger Institute and co-chair of the Human Cell Atlas Organising Committee
More information
*Previous Human Cell Atlas studies described in Nature Medicine and Cell have shown that cells in the nose and lung contain high levels of RNA for key proteins, the ACE2 receptor and the TMPRSS2 protease that allow the SARS-CoV-2 virus to enter cells.
**The Human Cell Atlas (HCA) is an international collaborative consortium which is creating comprehensive reference maps of all human cells—the fundamental units of life—as a basis for understanding human health and for diagnosing, monitoring, and treating disease. The HCA will impact every aspect of biology and medicine, propelling translational discoveries and applications and ultimately leading to a new era of precision medicine. The HCA was co-founded in 2016 by Dr Sarah Teichmann at the Wellcome Sanger Institute (UK) and Dr Aviv Regev, then at the Broad Institute of MIT and Harvard (USA). A truly global initiative, there are now more than 2,000 HCA members, from 75 countries around the world. https://www.humancellatlas.org
The HCA Oral & Craniofacial Biological Network is a group of scientists who collaborate to map the cells in the mouth and head. This group is coordinated by Kevin Byrd and Ines Sequeira.
Further HCA research on COVID at: https://www.humancellatlas.org/covid-19/
The data from this research is available at: https://www.covid19cellatlas.org
Publication:
Huang N, Perez P, et al. (2021) SARS-CoV-2 infection of the oral cavity and saliva. Nature Medicine. DOI: 10.1038/s41591-021-01296-8
Funding:
This research was supported by the NIDCR Division of Intramural Research, the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) grant DK034987 and the intramural programs of NIDDK, the National Cancer Institute, NIH Clinical Center, and the National Institute of Allergy and Infectious Diseases, and others. Please see the paper for further funders.