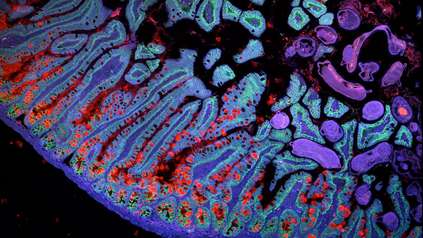
Human muscle map reveals how we try to fight effects of ageing
How muscle changes with ageing, and tries to fight its effects, is now better understood at the cellular and molecular level with the first comprehensive atlas of ageing muscles in humans.
Researchers from the Wellcome Sanger Institute and their collaborators at Sun Yat-sen University, China applied single-cell technologies and advanced imaging to analyse human skeletal muscle samples from 17 individuals across the adult lifespan. By comparing the results, they shed new light on the many complex processes underlying age-related muscle changes.
The atlas, published today (15 April) in Nature Ageing, uncovers new cell populations that may explain why some muscle fibres age faster than others. It also identifies compensatory mechanisms the muscles employ to combat ageing.
The findings offer avenues for future therapies and interventions to improve muscle health and quality of life as we age.
This study is part of the international Human Cell Atlas initiative to map every cell type in the human body1, to transform understanding of health and disease.
As we age, our muscles progressively weaken. This can affect our ability to perform everyday activities like standing up and walking. For some people, muscle loss worsens, leading to falls, immobility, a loss of autonomy and a condition called sarcopenia2. The reasons why our muscles weaken over time have remained poorly understood.
In this new study, scientists from the Wellcome Sanger Institute and Sun Yat-sen University, China used both single-cell and single-nucleus sequencing techniques3 along with advanced imaging to analyse human muscle samples from 17 individuals aged 20 to 75.
The team discovered that genes controlling ribosomes, responsible for producing proteins, were less active in muscle stem cells from aged samples. This impairs the cells’ ability to repair and regenerate muscle fibres as we age. Further, non-muscle cell populations within these skeletal muscle samples produced more of a pro-inflammatory molecule called CCL2, attracting immune cells to the muscle and exacerbating age-related muscle deterioration.
Age-related loss of a specific fast-twitch muscle fibre subtype, key for explosive muscle performance, was also observed4. However, they discovered for the first time several compensatory mechanisms from the muscles appearing to make up for the loss. These included a shift in slow-twitch muscle fibres to express genes characteristic of the lost fast-twitch subtype, and increased regeneration of remaining fast-twitch fibre subtypes.
The team also identified specialised nuclei populations3 within the muscle fibres that help rebuild the connections between nerves and muscles that decline with age. Knockout experiments in lab-grown human muscle cells by the team confirmed the importance of these nuclei in maintaining muscle function5.
“Our unbiased, multifaceted approach to studying muscle ageing, combining different types of sequencing, imaging and investigation reveals previously unknown cellular mechanisms of ageing and highlights areas for further study.”
Veronika Kedlian, first author of the study from the Wellcome Sanger Institute
“In China, the UK and other countries, we have ageing populations, but our understanding of the ageing process itself is limited. We now have a detailed view into how muscles strive to maintain function for as long as possible, despite the effects of ageing.”
Professor Hongbo Zhang, senior author of the study from Sun Yat-sen University, Guangzhou
“Through the Human Cell Atlas, we are learning about the body in unprecedented detail, from the earliest stages of human development through to old age. With these new insights into healthy skeletal muscle ageing, researchers all over the world can now explore ways to combat inflammation, boost muscle regeneration, preserve nerve connectivity, and more. Discoveries from research like this have huge potential for developing therapeutic strategies that promote healthier ageing for future generations.”
Dr Sarah Teichmann, senior author of the study from the Wellcome Sanger Institute, and co-founder of the Human Cell Atlas
More information
1. This study is part of the Human Cell Atlas (HCA), an international collaborative consortium which is creating comprehensive reference maps of all human cells—the fundamental units of life—as a basis for understanding human health and for diagnosing, monitoring, and treating disease. The HCA is likely to impact every aspect of biology and medicine, propelling translational discoveries and applications and ultimately leading to a new era of precision medicine.
The HCA was co-founded in 2016 by Dr Sarah Teichmann at the Wellcome Sanger Institute (UK) and Dr Aviv Regev, then at the Broad Institute of MIT and Harvard (USA). A truly global initiative, there are now more than -3,400 HCA members, from 101 countries around the world. https://www.humancellatlas.org
2. Sarcopenia is an age-related condition affecting approximately 25 per cent of older people and is significantly associated with frailty. The criteria for sarcopenia include low muscle mass, low physical function and low muscle strength. There are currently no approved medications to treat sarcopenia, and current advice is to remain physically active and eat healthily to prevent its onset.
3. Nuclei contain a cell’s genetic material. Because a muscle fibre consists of just one cell, but many nuclei, it brings unique challenges for study. Both single-cell RNA sequencing and single-nucleus RNA sequencing methods were used to address these challenges. While single-cell RNA sequencing looks at individual cells, including less common types of muscle stem cells and other supporting cells, profiling muscle fibres is difficult. Single-nucleus RNA sequencing, on the other hand, can focus on the multiple cell nuclei scattered throughout the muscle cell, to better explore its genetics.
4. Intercostal muscle biopsies of eight young – between 20-40 years old – and nine aged – between 60-75 years old – donors were provided by the Cambridge Biorepository for Translational Medicine.
5. Our muscles contain different types of muscle fibres: “slow-twitch” meant for endurance activities, while others are “fast-twitch” used for powerful, explosive movements. The researchers found that with ageing, one particular subtype of the fast-twitch muscle fibres is progressively lost, called type IIx.
Data sets
These data can be accessed here: https://www.muscleageingcellatlas.org
Publication
V.R. Kedlian et al. (2024) ‘Human skeletal muscle aging atlas’ Nature Aging. DOI: 10.1038/s43587-024-00613-3.
Funding
This research was supported by Wellcome and the National Key Research and Development Program of China. For full funding acknowledgements, please refer to the publication.