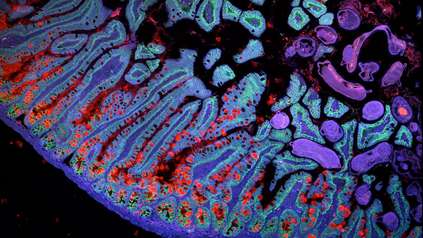
Cell mapping and ‘mini placentas’ give new insights into human pregnancy
For the first time, researchers have mapped the complete trajectory of placental development, helping shed new light on why pregnancy disorders happen.
Researchers from the Wellcome Sanger Institute, University of Cambridge, The Friedrich Miescher Institute for Biomedical Research (FMI), Switzerland, the European Molecular Biology Laboratory (EMBL), and collaborators, have created an in-depth picture of how the placenta develops and communicates with the uterus. This study is part of the Human Cell Atlas initiative1 that is mapping every cell type in the human body.
The paper, published today (29 March 2023) in Nature, details new information that was not possible to gain through previous methods and compares the findings to placental organoids, ‘mini-placentas’, that can be grown in the laboratory. This research informs and enables the development of experimental models of the human placenta.
The placenta is a temporary organ built by the foetus that facilitates vital functions such as foetal nutrition, oxygen and gas exchange, and protects against infections.
The formation and embedding of the placenta into the uterus, known as placentation, is crucial for a successful pregnancy. Understanding normal and disordered placentation at a molecular level can help answer questions about poorly understood disorders that include miscarriages, stillbirth, and pre-eclampsia.
In the UK, mild pre-eclampsia affects up to six per cent of pregnancies. Severe cases are rarer, developing in about one to two per cent of pregnancies2. Generally, many of the processes in pregnancy are not fully understood, despite pregnancy disorders causing illness and death worldwide. This is partly due to the process of placentation being difficult to study in humans, and while animal studies are useful, they have limitations due to physiological differences.
During its development, the placenta forms tree-like structures that attach to the uterus, and the outer layer of cells, called trophoblast, migrate through the uterine wall, transforming the maternal blood vessels to establish a supply line for oxygen and nutrients.
In new research, scientists built on previous studies investigating the early stages of pregnancy and applied cutting-edge single-cell genomics and spatial transcriptomics3 technologies to a rare historical set of samples4, capturing the process of placental development in unprecedented detail. These genomic techniques allowed researchers to see all of the cell types involved and how trophoblast cells communicate with the maternal uterine environment around them.
The team, from the Wellcome Sanger Institute and collaborators, uncovered the full trajectory of trophoblast development, suggesting what could go wrong in disease and describing the involvement of multiple populations of cells, such as maternal immune and vascular cells.
The team also compared these results to placental trophoblast organoids, sometimes called ‘mini-placentas’, that are grown in the lab. They found that most of the cells identified in the tissue samples can be seen in these organoid models. Some later populations of trophoblast are not seen and are likely to form in the uterus only after receiving signals from maternal cells.
The team focussed on the role of one understudied population of maternal immune cells known as macrophages. They also discovered that other maternal uterine cells release communication signals that regulate placental growth.
The insights from this research can start to piece together the unknowns about this stage of pregnancy. The new understanding will help in the development of effective lab models to study placental development and facilitate new ways to diagnose, prevent, and treat pregnancy disorders.
“Despite the placenta being a vital organ that plays an important role in everyone’s life, its development is poorly understood. Although we have previously seen how the structural cells of the placenta, trophoblast cells, attach and start to travel through the maternal uterus, the existing resources and models have limited further understanding. For the first time, we have been able to draw the full picture of how the placenta develops and describe in detail the cells involved in each of the crucial steps. This new level of insight can help us improve laboratory models to continue investigating pregnancy disorders, which cause illness and death worldwide.”
Anna Arutyunyan, co-first author from the Wellcome Sanger Institute and the University of Cambridge
“Using a systems biology approach, this work captures and describes the specialised placental extravillous trophoblast cells as they implant into the maternal uterus during early pregnancy in humans. It provides an essential resource that will help improve our understanding of the maternal-foetal interactions that are critical for a successful pregnancy. Our knowledge about early placentation in humans is limited. Only with the combination of expertise from computational biology, human reproduction, organoids and stem cell model systems, and with the use of historical and rare pregnant hysterectomy samples, has it been possible to shed light on the processes occurring during this critical time that determines pregnancy outcome.”
Dr Margherita Yayoi Turco, co-senior author from the Friedrich Miescher Institute for Biomedical Research (FMI) in Basel, Switzerland
“Studying pregnancy in humans is difficult, but is necessary if we are to help prevent and treat disorders that arise throughout gestation. This research is unique as it was possible to use rare historical samples that encompassed all the stages of placentation occurring deep inside the uterus. We are glad to have created this open-access cell atlas to ensure that the scientific community can use our research to inform future studies.”
Professor Ashley Moffett, co-senior author from the University of Cambridge
“Successful placental implantation is an important step in pregnancy, and many pregnancy disorders, such as pre-eclampsia, can be linked to issues in its development. Insights from our research show that our previous understanding of placental implantation was incomplete and that the maternal uterine cells release communication signals to encourage placental growth. Understanding the communication signals through spatial data gives us a fuller picture of how the two organs, the placenta and the uterus, work together highlighting where issues could arise in disease.”
Dr Roser Vento-Tormo, co-senior author from the Wellcome Sanger Institute
More information
- This study is part of the international Human Cell Atlas (HCA) consortium, which is aiming to map every cell type in the human body as a basis for both understanding human health and for diagnosing, monitoring, and treating disease. An open, scientist-led consortium, HCA is a collaborative effort of researchers, institutes, and funders worldwide, with more than 2,700 members from 89 countries across the globe. More information can be found at https://www.humancellatlas.org/
- Pre-eclampsia statistics, Tommy’s. Available at https://www.tommys.org/pregnancy-information/pregnancy-complications/pre-eclampsia-information-and-support [Accessed March 2023]
- Spatial transcriptomics looks at the gene expression of the cell and its surroundings in the body. Combines with single-cell sequencing data, it enables researchers to see what a cell is and how it is interacting with the environment around it, including communication events and responses to immune signals.
- This research used a unique historical collection of samples of uteruses from >30 years ago, from first trimester hysterectomies. The samples were collected and stored by Professor Ashley Moffett at the University of Cambridge. The researchers would like to thank and acknowledge the organ donors and their families.
Publication:
A. Arutyunyan, K. Roberts, K. Troulé, et al. (2023) ‘Spatial multiomics map of trophoblast development in early pregnancy.‘ Nature. DOI: 10.1038/s41586-023-05869-0
Funding:
This research was funded by Wellcome, The Royal Society, and the European Research Council. A full acknowledgement list can be found on the publication.