Establishing the molecular blueprint of early embryo development
A pioneering group of biologists, physicists and mathematical modellers in Cambridge have studied the genetic activity of over 100,000 embryonic cells to establish the molecular blueprint of mouse early embryo development. This new research provides fundamentally important information on how mammalian embryos develop during gastrulation, a key stage of development, and paves the way for new understanding of the earliest stages of life.
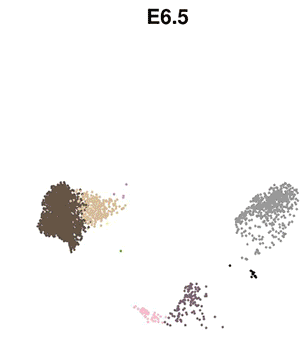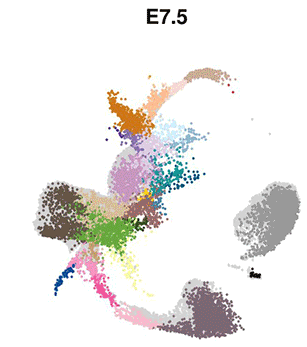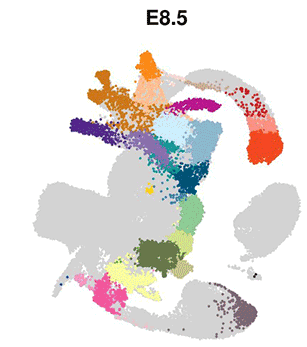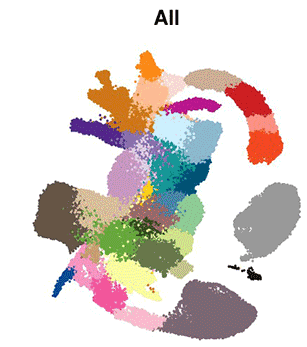 The eminent biologist Lewis Wolpert famously stated that “It’s not birth, marriage or death, but gastrulation that is truly the most important time in your life”. Gastrulation represents the process in animal (and human) development whereby precursor cells in the embryo become genetically programmed to generate all of the different organs in the body, including the brain, heart, lungs, gut and muscles. Gastrulation is a critically important step in embryo development, however, detailed understanding of this process at the molecular level has, until now, been limited.
The eminent biologist Lewis Wolpert famously stated that “It’s not birth, marriage or death, but gastrulation that is truly the most important time in your life”. Gastrulation represents the process in animal (and human) development whereby precursor cells in the embryo become genetically programmed to generate all of the different organs in the body, including the brain, heart, lungs, gut and muscles. Gastrulation is a critically important step in embryo development, however, detailed understanding of this process at the molecular level has, until now, been limited.
In a landmark study published in Nature, Cambridge scientists have generated the first comprehensive molecular map of gastrulation in mammals. Measuring the genetic activity in 116,312 single cells within the mouse embryo between 6.5 to 8.5 days after fertilisation, the researchers have established the molecular blueprint for mammalian embryonic development.
The researchers used cutting-edge single-cell technology to measure which genes are activated in the mouse embryo across a number of sequential time-points. Beginning with only a small number of distinct cell-types detected at the start of gastrulation, the cells branch out into over 30 different cell types with different genetic profiles, all within a time span of only 48 hours.
Computational analyses enabled the scientists to generate “interactive maps” where each cell is represented by a dot, and cells with similar molecular profiles are positioned close to each other. These new maps, which are freely available online for other researchers to use, illustrate the trajectories of cellular development from one cell type to the next, and show the precise genetic processes that enable all of the cells and organs of the body to develop from their early embryonic origins.
“Our new map provides a molecular blueprint to outline how embryonic development proceeds under normal conditions. It allows us to see which different sets of genes are activated when unspecified embryonic cells proliferate and diversify into all the specific cell types across the body. The map is also an invaluable reference point to understand how genetic mutations can disrupt embryo growth and cause developmental disorders and diseases.”
Professor Bertie Göttgens Group leader at the Wellcome – MRC Cambridge Stem Cell Institute
The researchers tested the new molecular map by investigating Tal1, a gene that is essential for normal blood development, yet if activated in the wrong cells, can cause leukaemia. By comparing the reference atlas to over 10,000 Tal1 mutant cells, the researchers were able to decipher the consequences of the Tal1 genetic mutation.
“To investigate the role of Tal1 in blood development, we mixed embryonic stem cells carrying the mutated Tal1 gene with very early normal embryos to make chimaeras. Because these chimaeras could still make blood from their normal cells, we were able to study the mutant cells in their proper biological context.”
Professor Jenny Nichols Group leader at the Wellcome – MRC Cambridge Stem Cell Institute
“By comparing our experimental data with the data collated within the molecular map, we were able to decipher precisely what was going on within the cells with mutant Tal1 genes. We could see that the mutant cells weren’t simply getting stuck, or deciding to become a different cell-type, but instead the cells started expressing a wide range of different genes, as if they were confused about what cell type they should mature into.”
Dr John Marioni Group leader at EMBL’s European Bioinformatics Institute (EMBL-EBI) and the Cancer Research UK Cambridge Institute, and Associate Faculty at the Wellcome Sanger Institute
Investigating the genetic basis of blood development is just one way the new molecular map could be used to understand normal and disease processes. The extensive nature of the map, which contains molecular information on all of the developing cell types in the embryo, will allow other researchers to gain a deeper understanding of how a whole range of organs develop. This in turn will enable scientists to develop new protocols for the production of authentic cell types for drug screening, as well as therapies aiming to regenerate diseased or ageing organs.
“By piecing together this ‘molecular map’ of how cells develop in a mouse embryo, the researchers have created an important and useful resource for the research community. Science is a collaborative effort and this team of biologists, physicists and mathematicians have been able to gain new understanding of gastrulation, a pivotal process in development which generates the three cell layers that give rise to all the organs of the body.”
Dr Sheny Chen from Wellcome’s Cellular and Developmental Sciences team
More information
Publication
Pijuan-Sala, B et al. A single-cell molecular map of mouse gastrulation and early organogenesis. Nature 2019; published online DOI: 10.1038/s41586-019-0933-9
The support of Wellcome, the Medical Research Council, Bloodwise, Cancer Research UK, National Institutes of Health*, and the European Molecular Biology Laboratory are gratefully acknowledged by the team.
* Research reported in this press release was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health under award number 1 R24 DK106766-01A1. The NIDDK supported $5,000 worth of work, against a total project cost of $800,000.
Funding
This work was funded by Wellcome, the Medical Research Council, Cancer Research UK, Bloodwise and the National Institutes of Health.
Selected websites
The Wellcome - MRC Cambridge Stem Cell Institute
The Wellcome – MRC Cambridge Stem Cell Institute is a world-leading centre for stem cell research with a mission to transform human health through a deep understanding of normal and pathological stem cell behaviour. Bringing together biological, clinical and physical scientists operating across a range of tissue types and at multiple scales, we explore the commonalities and differences in stem cell biology in a cohesive and inter-disciplinary manner. In 2018, we will relocate to a new purpose-built home on the Cambridge Biomedical Campus. Housing over 350 researchers, including a critical mass of clinician scientists, the Institute will integrate with neighbouring disease-focused research institutes and also act as a hub for the wider stem cell community in Cambridge. www.stemcells.cam.ac.uk
The European Bioinformatics Institute (EMBL-EBI)
The European Bioinformatics Institute (EMBL-EBI) is a global leader in the storage, analysis and dissemination of large biological datasets. We help scientists realise the potential of ‘big data’ by enhancing their ability to exploit complex information to make discoveries that benefit humankind. We are at the forefront of computational biology research, with work spanning sequence analysis methods, multi-dimensional statistical analysis and data-driven biological discovery, from plant biology to mammalian development and disease. We are part of EMBL and are located on the Wellcome Genome Campus, one of the world’s largest concentrations of scientific and technical expertise in genomics. www.ebi.ac.uk
Cancer Research UK Cambridge Institute
Cancer Research UK Cambridge Institute combines world leading basic cancer biology with key technologies to bear on practical questions of cancer diagnosis, treatment and prevention.
Research in the Institute focuses primarily on Tumour Ecology and Evolution. The core funding provided by Cancer Research UK allows each of our Group Leaders the freedom to expand their programme of work and follow innovative new ideas. The CRUK Cambridge Institute is based on the Cambridge Biomedical Campus – Europe’s largest centre of health science and medical research – providing unique access to both the clinic and industry. By working in close alignment with the Cancer Research UK Cambridge Centre, researchers within the Institute can gain from clinical trial infrastructure, additional funding streams as well as networking opportunities. www.cruk.cam.ac.uk
Babraham Institute
The Babraham Institute undertakes world-class life sciences research to generate new knowledge of biological mechanisms underpinning ageing, development and the maintenance of health. Our research focuses on cellular signalling, gene regulation and the impact of epigenetic regulation at different stages of life. By determining how the body reacts to dietary and environmental stimuli and manages microbial and viral interactions, we aim to improve wellbeing and support healthier ageing. The Institute is strategically funded by the Biotechnology and Biological Sciences Research Council (BBSRC), part of UK Research and Innovation, through an Institute Core Capability Grant and also receives funding from other UK research councils, charitable foundations, the EU and medical charities. https://www.babraham.ac.uk/
Wellcome Sanger Institute
The Wellcome Sanger Institute is one of the world’s leading genome centres.
Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease.
Find out more at www.sanger.ac.uk or follow @sangerinstitute on Twitter, Facebook, LinkedIn and on our Blog.
About Wellcome
Wellcome exists to improve health by helping great ideas to thrive. We support researchers, we take on big health challenges, we campaign for better science, and we help everyone get involved with science and health research. We are a politically and financially independent foundation. wellcome.org
The Medical Research Council
The Medical Research Council is at the forefront of scientific discovery to improve human health. Founded in 1913 to tackle tuberculosis, the MRC now invests taxpayers’ money in some of the best medical research in the world across every area of health. Thirty-two MRC-funded researchers have won Nobel prizes in a wide range of disciplines, and MRC scientists have been behind such diverse discoveries as vitamins, the structure of DNA and the link between smoking and cancer, as well as achievements such as pioneering the use of randomised controlled trials, the invention of MRI scanning, and the development of a group of antibodies used in the making of some of the most successful drugs ever developed. Today, MRC-funded scientists tackle some of the greatest health problems facing humanity in the 21st century, from the rising tide of chronic diseases associated with ageing to the threats posed by rapidly mutating micro-organisms. www.mrc.ac.uk
Cancer Research UK
Cancer Research UK is the world’s leading cancer charity dedicated to saving lives through research. The Charity receives no government funding for its life-saving research. Every step it makes towards beating cancer relies on every donation made. Cancer Research UK has been at the heart of the progress that has already seen survival in the UK double in the last 40 years. Today, 2 in 4 people survive their cancer for at least 10 years. Cancer Research UK’s ambition is to accelerate progress so that by 2034, 3 in 4 people will survive their cancer for at least 10 years. Cancer Research UK supports research into all aspects of cancer through the work of over 4,000 scientists, doctors and nurses. Together with its partners and supporters, Cancer Research UK’s vision is to bring forward the day when all cancers are cured. www.cancerresearchuk.org
Bloodwise
Bloodwise is the UK’s specialist blood cancer research charity dedicated to improving the lives of people living with and beyond blood cancer. The charity, which was formed in 1960, changed its name from Leukaemia & Lymphoma Research in September 2015. The charity’s research is targeted at understanding more about blood cancer, finding causes, improving diagnosis and treatments, and running ground-breaking clinical trials for patients. The charity champions patients’ needs by influencing relevant decision makers and influencers, and seeking to raise awareness of the issues faced by patients. Their patient services provide information, support and assistance to patients at every stage of their journey. www.bloodwise.org.uk


