Blood counts are clues to human disease
A new genome-wide association study published today in Nature Genetics begins to uncover the basis of genetic variations in eight blood measurements and the impact those variants can have on common human diseases. Blood measurements, including the number and volume of cells in the blood, are routinely used to diagnose a wide range of disorders, including anaemia, infection and blood cell cancers.
An international team of scientists measured haemoglobin concentration, the count and volume of red and white cells and the sticky cells that prevent bleeding – platelets, in over 14,000 individuals from the UK and Germany. They uncovered 22 regions of the human genome implicated in the development of these blood cells. Of the 22 regions, 15 had not previously been identified.
The study represents the first genome-wide association of blood measurements to be completed in cohorts with large sample sizes.
“This study has been made possible by a great collaboration of scientists from the UK and Germany, and the contribution of clinical colleagues working in the field of heart disease, diabetes and coeliac disease in the UK, Germany and the United States and Finland. This unique collaboration has allowed us to discover novel genetic determinants of blood cell parameters, providing important insights into novel biological mechanisms underlying the formation of blood cells by the blood stem cells and their role in disease.
“This study highlights the importance of studying large collections of samples from healthy individuals where many different traits are measured.”
Dr Nicole Soranzo Group leader at the Wellcome Trust Sanger Institute and co-lead of the HaemGen consortium
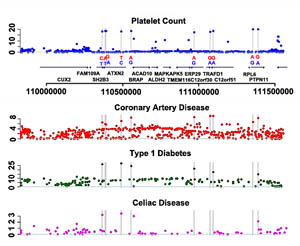
The team compared regions of the human genome implicated in blood cell development with regions associated with risk of heart disease. By looking at the genetic data of 10,000 people with disease with that of 10,000 apparently healthy people, they found that one of the genetic variants associated with platelet counts also causes an increased risk of heart disease. The new variant was found in a region of the genome already known to influence the risk of hypertension, coeliac disease and diabetes in children and young adults, or so-called type 1 diabetes.
Further analysis showed that these genetic risk factors are uniquely found in individuals of European origin. By comparing human data with genetic data from chimpanzees, the team were able to conclude that the genetic variant was the result of a selection event favouring variants that increase the risk of heart disease, coeliac disease and type 1 diabetes in European populations 3,400 years ago. The authors suggest that the risk factors were positively selected for because they gave carriers an increased protection against infection.
“The study of blood traits is challenging because of the difficulty of teasing apart biological processes underlying the origin of blood cells. Until now, few genome-wide association studies have looked beyond single traits. But, through a systematic analysis of correlated traits we can begin to discover such shared genetic variants, forming the basis for understanding how these processes interact to influence health and disease.
“Using these techniques, we can now begin to understand the complex genetic basis of a whole variety of human diseases.”
Dr Christian Gieger Head of the Genetic Epidemiology research unit at the Helmholtz Zentrum and co-lead of the HaemGen consortium
Scientists at the Wellcome Trust Sanger Institute, UK and the Helmholtz Zentrum Munich, Germany initiated the European HaemGen consortium, which encompasses groups from the UK (TwinsUK-KCL, NHS Blood and Transplant (NHSBT), University of Cambridge and University of Leicester) and Germany (Study of Health in Pomerania (SHIP) in Greifswald, the KORA study in the region of Augsburg and GerMIFS (University of Lübeck and Regensburg)). The HaemGen consortium aims to identify genetic loci contributing to variation in blood measurements and uncovers the potential correlation of these loci with disease phenotypes.
“We have uncovered a novel variant linking platelet counts with heart attacks. Further characterisation of the regions uncovered in this study has the potential to improve our understanding how blood cell development is linked with human diseases, including blood cell cancers.”
Dr Nicole Soranzo Sanger Institute
Dr Soranzo has an honorary appointment at the Department of Twin Research, Kings College and is also joint lead author on a second study into haematological parameters published on the same day in Nature Genetics.
More information
Funding
This work was supported by the Wellcome Trust, the European Union and the National Institute for Health Research (NIHR).
Participating Centres
A full list of participating centres is available at the Nature Genetics website.
Publications:
Selected websites
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute, which receives the majority of its funding from the Wellcome Trust, was founded in 1992. The Institute is responsible for the completion of the sequence of approximately one-third of the human genome as well as genomes of model organisms and more than 90 pathogen genomes. In October 2006, new funding was awarded by the Wellcome Trust to exploit the wealth of genome data now available to answer important questions about health and disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.


