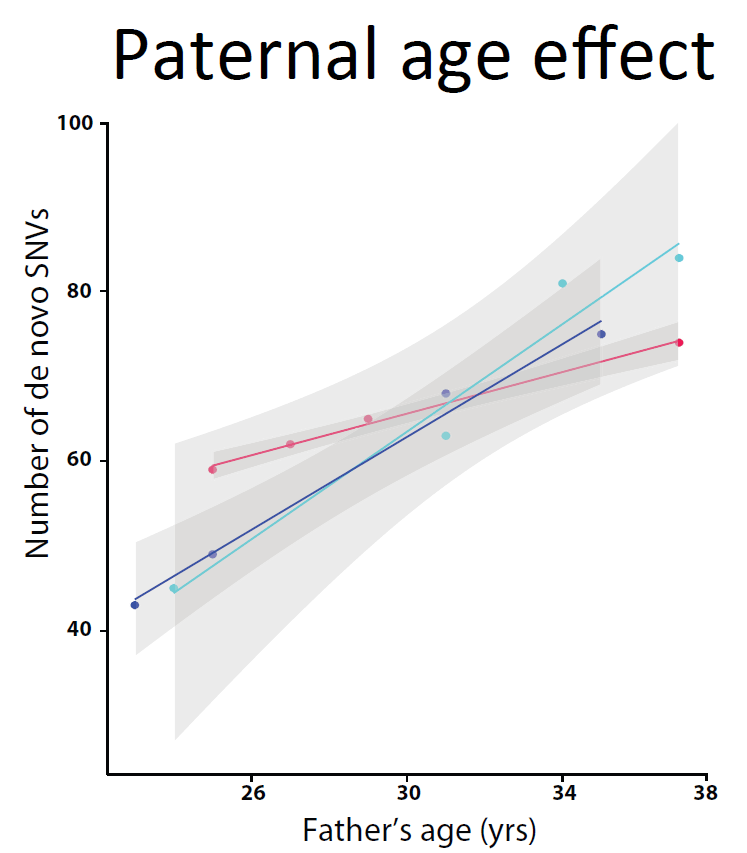
Sperm mutation rate varies between fathers
Researchers compared mutation rates in sperm and eggs for multi-sibling families, confirming that fathers contribute more mutations to their children than mothers. They revealed for the first time that the rate at which mutations in sperm accumulate with age varies from father to father.
The study has important medical implications for predicting the risk of a second child inheriting a disease-causing mutation from its parents. It also shows that developing sperm and egg cells undergo the same mutational processes as cancer cells, which may help researchers understand the origin of some cancer causing mutations.
Errors in DNA copying during cell division and development can cause new mutations — called de novo mutations — at any time from the moment of conception. Mutations that occur in the germ line — the cells that develop into sperm or eggs — can be passed on to the next generation and, perhaps, cause disease in children.
The researchers examined three families with at least four children and sequenced DNA from their blood cells. By comparing these genome sequences, the team could infer which mutations were inherited from either the mother or father’s germ cells and look back in time to see when they had occurred.
“Because parents get older as successive children are born, we can see how the age of the parent affects the number of de novo mutations. Older parents pass on more mutations. We found an average of 2.9 new mutations per year of the father’s life but it varies among families, from more than three mutations to less than one a year. Something is happening systematically in the paternal germ line that we just didn’t know about.
“It might be that some men pass on more mutations because they have an intrinsically higher mutation rate every time that DNA is copied, or because turnover in the cells that form their sperm is higher, with DNA being copied more often.”
Dr Raheleh Rahbari Joint first author on the study, from the Wellcome Trust Sanger Institute
By looking at DNA from parents’ blood, the team could see what proportion of cells carried any given mutation. Very little is known about the timing, rates or types of mutations that occur in germ cells: this study provides the first experimental results of this important period, results that can inform clinical decisions.
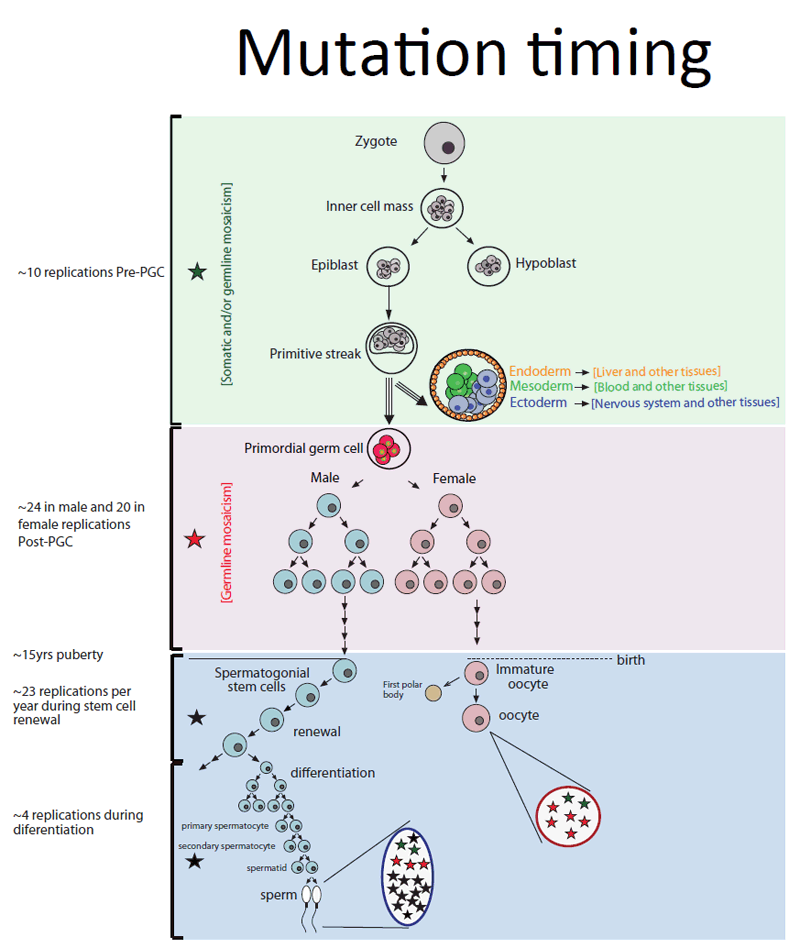
Approximately 4 per cent of new mutations passed on to children were also found in a small proportion of a parent’s blood cells. These are classed as mosaic mutations, and are likely to have occurred very early on in the parent’s development, before the embryo was about 4000 cells and before the cells that form blood and germ cells divided into separate lineages.
“If a mutation occurs very early in development, then many cells including sperm, eggs and blood cells, will carry it: if it occurs later, then fewer cells will. This has dramatic consequences for the risk of a second child sharing a mutation found in an older sibling. By examining the DNA in parents’ blood, we should be able to predict that risk more accurately.”
Dr Matt Hurles Senior author on the paper from the Sanger Institute
The average risk of a second child inheriting the same de novo mutation carried by an older sibling is only about one in one hundred, but this rises to nearly one in 12 if the mutation is found in 1 per cent of parental blood cells and one in six if in more than six per cent of parents blood cells. These mosaic de novo mutations are derived equally from the mother and from the father.
Knowing what proportion of a parents cells is affected may significantly improve clinical advice to parents of a child with a genetic condition who are thinking of having more children.
This study also showed almost all germline mutations resulted from two distinct underlying mutation processes. These same two mutational processes are also found in most cells in the body and are thought to arise when a cell fails to repair mistakes in its DNA. Their regular, clock-like accumulation over time means that the number of mutations correlates with age. Sharing of these signatures between cancers and germ cells indicates that fundamental cellular processes that operate in cancers also operate in germ cells, and may help researchers understand the origin of some cancer causing mutations.
The types of mutation are shared by both men and women alike and the pattern remains unchanged through sperm or egg development, even though these two cell types follow very different paths: a woman is born with all her eggs as immature cells; by contrast, male germ cells undergo division throughout life before becoming mature sperm.
However, the numbers of mutations contributed by each parent differs markedly during life: In the mother, most mutations arise in the development of immature germ cells, while the father contributes most mutations during the unrelenting cell division after puberty, as mature sperm are formed.
“Through analysing adult blood samples, we have an exciting glimpse of the mutation processes that operate as we develop from a single, fertilised egg to an adult consisting of trillions of cells. Developing our appreciation of these distant events in the germline may shed light on the development of neoplastic cancer cells and will help us to understand the risk of disease and, perhaps, to better help patients and their families.”
Professor Sir Mike Stratton Director of the Sanger Institute and an author on the study
More information
Funding
This research was funded by the Wellcome Trust (grant WT098051). Generation Scotland has received core funding from the Chief Scientist Office of the Scottish Government Health Directorates CZD/16/6 and the Scottish Funding Council HR03006. Funding for UK10K was provided by the Wellcome Trust under award WT091310.
Participating centres
- Wellcome Trust Sanger Institute, Hinxton, UK.
- Department of Human Genetics, Genentech, Inc., South San Francisco, California, USA.
- Department of Bioinformatics and Computational Biology, Genentech, Inc., South San Francisco, California, USA.
- Institute of Cardiovascular and Medical Sciences, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, UK.
- Medical Research Institute, University of Dundee, Dundee, UK.
- Institute of Genetics and Molecular Medicine, University of Edinburgh, Edinburgh, UK.
- UK10K consortium, see paper for full details.
Publications:
Selected websites
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute is one of the world’s leading genome centres. Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.


