Analysis of horse pathogen sheds light on persistent infections
Analysis of an ancient pathogen of horses shows that it went through a major population replacement during the global conflicts of the late 19th and early 20th centuries and has shed light on the genetics of low diversity and persistent infections such as human immunodeficiency virus (HIV) and tuberculosis.
In the largest-ever study of the bacteria Streptococcus equi, which causes the disease strangles in horses, researchers were surprised by the genetic similarity of the 224 samples they had procured from horses across the globe.
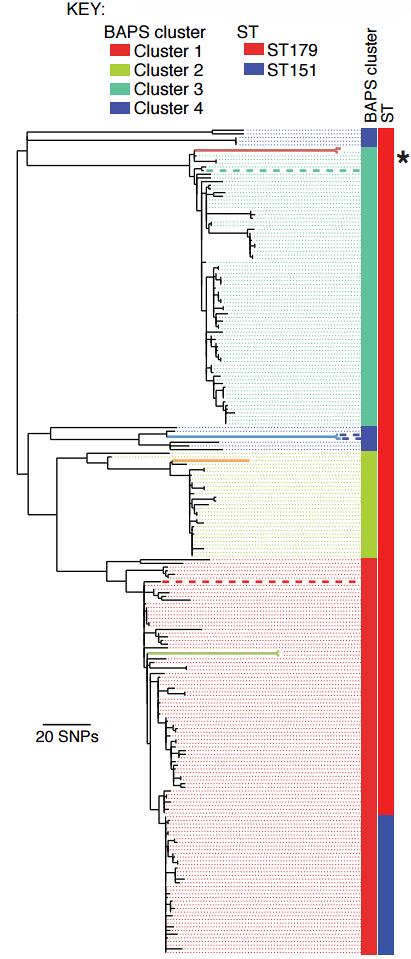
“The mobilisation and mixing of horses in conflicts such as WWI provided perfect conditions for Streptococcus equi to thrive. This combined with the high mortality rates among the horses and the subsequent intense breeding that produced a new naïve host population could explain why we see so little diversity in modern Streptococcus equi strains.”
Simon Harris First author from the Wellcome Trust Sanger Institute
While loss of diversity could be considered detrimental to bacterial populations, Streptococcus equi still infects more than 20,000 horses in the UK alone each year. Its success, researchers believe, is due to the evolutionary niche it has carved out for itself in horses, where it first causes an acute infection and then persists in the horse for years while showing no visible signs.
Researchers observed gene decay in the persistent samples they sequenced; this is where bacteria shed genes that are no longer useful. In the case of Streptococcus equi living in one horse for long periods, genes that helped the bacteria spread to new hosts and share genetic material with other bacteria became less useful. At the same time, Streptococcus equi adapted to its environment in horses, becoming able to evade the immune system so effectively that more than one strain can infect the same horse and the persistent bacteria is able to resurface, causing multiple periods of painful disease.
“Strangles is a distressing disease that causes large abscesses to form in a horse’s head and neck. The data we have gathered in this study will enable us to pinpoint the genes that help the bacteria to persist, spread and thrive in the horse population. Our hope is that these genes will be effective vaccine targets.”
Andrew Waller Head of Bacteriology at the Animal Health Trust
The ability of Streptococcus equi to adapt to living in a persistent state within its host and still infect new horses mirrors the situation with HIV. Researchers hypothesise that in both cases the original genetic sequence that infected the host is more often passed on to the next host. The bacteria or viruses with these ancestral DNA sequences then change and adapt to persist in the new host. This, scientists believe is why diversity appears so low in these populations.
Further parallels can be drawn between Streptococcus equi and the bacteria that causes tuberculosis. The population of bacteria responsible for this respiratory disease in humans also has low diversity between patients but is able to quickly develop antibiotic resistance within its host.
“Unravelling the complex population dynamics of Streptococcus equi sheds new light on the balancing act between acute and persistent infection that is going on in many pathogens. Not only does this collection of whole-genome sequences for Streptococcus equi offer hope for an effective strangles vaccine, it also provides us with a useful model for understanding persistent infection in humans.”
Matthew Holden A senior author from the Wellcome Trust Sanger Institute and the University of St Andrews
“The Horserace Betting Levy Board is delighted to have supported this important work. Streptocuccus equi is a persistent challenge to the horse population worldwide and this significant progress towards an effective vaccine is extremely good news. We are pleased too that this programme may also have useful implications for such devastating human diseases as HIV and TB. We look forward to seeing what happens next.”
Annie Dodd Grants Manager at the Horserace Betting Levy Board
More information
Funding
The authors would like to thank the Horserace Betting Levy Board for funding the analysis of the eqbE mutant (ref Vet/prj/758). The Horse Trust (www.horsetrust.org.uk) funded the collection of isolates from UK outbreaks of strangles. This research was also supported by the Wellcome Trust, grant 098051.
Publications:
Selected websites
The Horse Trust
Established in 1886, The Horse Trust is the world’s oldest equine charity, funding high quality non-invasive veterinary research that positively impacts the health of the wider equine population. The Horse Trust also provides retirement for some of the UK’s working horses, care for horses that have experienced neglect and training for equine industry professionals.
Animal Health Trust
Everything the Animal Health Trust does has the health and welfare of animals at its heart. We are a veterinary charity, based in Suffolk, which offers clinical referral services and diagnostic testing for horses, dogs and cats in East Anglia, across the UK and internationally. Through our research programmes, we develop new diagnostic tests, treatments and vaccines to help thousands of animals. Our commitment to education ensures the knowledge we gain is shared internationally to benefit animals all around the world.
University of St Andrews
Over the last 600 years, the University of St Andrews has established a reputation as one of the world’s leading research and teaching centres. Today, we offer a flexible degree structure based on your choice of subject specialism or research, creating an environment which nurtures inquisitive minds and a culture of shared learning.
Website
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute is one of the world's leading genome centres. Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.


