Novel pathogen epidemic identified in sub-Saharan Africa
A new study out today (Sunday 30 September) reveals that the emergence and spread of a rapidly evolving invasive intestinal disease, that has a significant mortality rate (up to 45 per cent) in infected people in sub-Saharan Africa, seems to have been potentiated by the HIV epidemic in Africa.
The team found that invasive non-Typhoidal Salmonella (iNTS) disease is caused by a new form of the bacteria Salmonella Typhimurium that has spread from two different focal hubs in Southern and Central Africa beginning 52 and 35 years ago, respectively. They also found that one of the major contributing factors for the successful spread of iNTS was the acquisition of genes that afford resistance to several front line drugs used to treat blood-borne infection such as iNTS.
iNTS is a blood-borne infection that kills approximately one of four people in sub-Saharan Africa who catch it. Yet, in the rest of the world, NTS is a leading cause of acute inflammatory diarrhoea that is self-limiting and tends to be fatal in less than 1 per cent of people infected. The disease is more severe in sub-Saharan Africa than the rest of the world because of factors such as malnutrition, co-infection with malaria or HIV and potentially the novel genotype of the Salmonella bacteria.
“The immune system susceptibility provided by HIV, malaria and malnutrition at a young age, may provide a population in sub-Saharan Africa that is large enough for this detrimental pathogen to enter, adapt, circulate and thrive. We used whole genome sequencing to define a novel lineage of Salmonella Typhimurium that is causing a previously unrecognised epidemic across the region. Its genetic makeup is evolving into a more typhoid like bacteria, able to efficiently spread around the human body.”
Chinyere Okoro Joint first author from the Wellcome Trust Sanger Institute
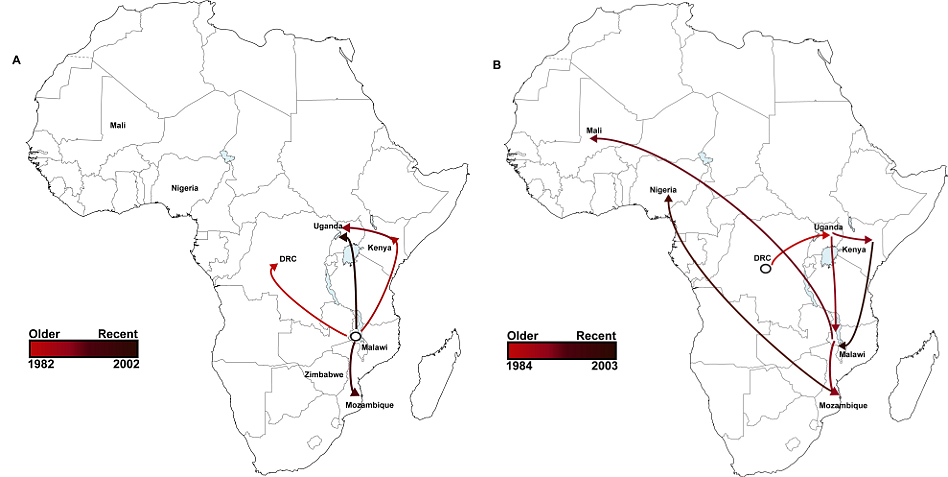
From sequenced samples, the team created a phylogenetic or ‘family tree’, depicting the pathogen’s evolution, dating when each sample first emerged and overlaying this with geographical information about where these samples came from. They found that this invasive disease comprises of two very closely related waves; the first wave originated from a possible south-eastern hub, about 52 years ago and the second originated about 35 years ago, possibly from the Congo Basin.
“The HIV epidemic in sub-Saharan Africa is thought to have begun in a central region and underwent expansion eastwards, a strikingly similar dynamic to that observed for second iNTS wave. Our findings suggest the current epidemic of iNTS and its transmission across sub-Saharan Africa may have been potentiated by an increase in the critical population of susceptible, immune-compromised people.”
Dr Robert Kingsley Joint first author from the Wellcome Trust Sanger Institute
The team identified that the vast majority of samples from the second wave of iNTS contains a gene that makes them resistant to chloramphenicol, a frontline antibiotic in the treatment of Salmonella. This gene was not present in the samples from the first wave of iNTS. This observation suggests that iNTS acquired this gene early on in the evolution of the second wave, probably around the time of its spread from the Congo basin.
“Because it acquired resistance to chloramphenicol, this pathogen has much greater opportunity to survive and spread across the region. This is the first time that the power of whole-genome sequencing has been used to track the spread of iNTS. Our research highlights the power this approach has to monitor the emergence and spread of dangerous pathogens both locally and globally over time.
“There has been some evidence that this disease can be passed from human to human. Now the race is on to discover how NTS is actually transmitted in sub-Saharan Africa so that effective intervention strategies can be implemented.”
Professor Gordon Dougan Lead author from the Wellcome Trust Sanger Institute
More information
Funding
This work was funded by the Wellcome Trust and Tropical Research Fellowship from the Wellcome Trust and Clinical Research Fellowship from GlaxoSmithKline.
Participating Centres
- Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton Cambridge, UK. CB10 1SA
- Mahidol-Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
- Department of Clinical Infection, Microbiology and Immunology, Institute for Infection and Global Health, University of Liverpool, Liverpool, UK
- Centre for Microbiology Research, Kenya Medical Research Institute, Nairobi, Kenya
- Malawi-Liverpool-Wellcome Trust Clinical Research Program, University of Malawi College of Medicine, Blantyre, Malawi
- Department of Microbiology, College of Medicine, University of Malawi, Blantyre, Malawi
- Department of Gastroenterology, Institute of Translational Medicine, Liverpool University, Liverpool, UK. L69 3GE
- Health Protection Agency, Laboratory for Gastrointestinal Infections, Centre for Infections, London, United Kingdom
- Norwich Medical School, University of East Anglia, Norwich, United Kingdom
- Cellular and Molecular Medicine, School of Medical Sciences, University of Bristol, Bristol, United Kingdom
- Division of Paediatric Infectious Diseases, Department of Paediatrics and Human Development, Michigan State University, East Lansing, MI 48824, USA
- National Hospital Abuja, Plot 132 Central District (Phase II), Garki Abuja, Nigeria
- Barcelona Centre for International Health Research (CRESIB, Hospital Clínic-Universitat de Barcelona), Barcelona, Spain
- Centro de Investigação em Saúde de Manhiça (CISM), Manhiça, Mozambique
- Instituto Nacional de Saúde, Ministerio de Saúde, Maputo, Mozambique
- Novartis Vaccines Institute for Global Health S.r.l. (NVGH), Via Fiorentina 1, 53100 Siena, Italy
- MRC Centre for Immune Regulation, School of Immunity and Infection, College of Medicine and Dental Sciences, University of Birmingham, Birmingham, B15 2TT, UK.
- Center for Vaccine Development, University of Maryland, Baltimore, HSFI 480, 685 West Baltimore St., Baltimore, MD 21201, USA
- Department of Medicine, University of Maryland, Baltimore, HSFI 480, 685 West Baltimore St., Baltimore, MD 21201, USA
Publications:
Selected websites
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute is one of the world’s leading genome centres. Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.