The genetic basis of brain diseases
In research published today, scientists have studied human brain samples to isolate a set of proteins that accounts for over 130 brain diseases. The paper also shows an intriguing link between diseases and the evolution of the human brain.
Brain diseases are the leading cause of medical disability in the developed world according to the World Health Organisation and the economic costs in the USA exceeds $300 billion.
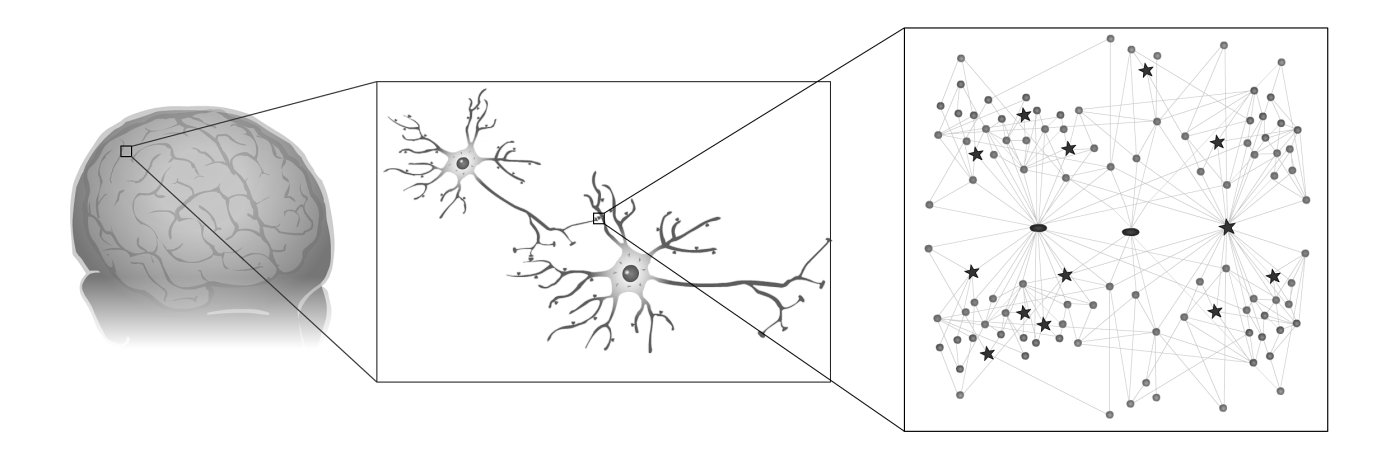
The brain is the most complex organ in the body with millions of nerve cells connected by billions of synapses. Within each synapse is a set of proteins, which, like the components of an engine, bind together to build a molecular machine called the postsynaptic density – also known as the PSD. Although studies of animal synapses have indicated that the PSD could be important in human diseases and behaviour, surprisingly little was known about it in humans.
A team of scientists, led by Professor Seth Grant at the Wellcome Trust Sanger Institute and Edinburgh University, have extracted the PSDs from synapses of patients undergoing brain surgery and discovered their molecular components using a method known as proteomics. This revealed that 1461 proteins, each one encoded by a different gene, are found in human synapses. This has made it possible, for the first time, to systematically identify the diseases that affect human synapses and provides a new way to study the evolution of the brain and behaviour.
“We found that over 130 brain diseases involve the PSD – far more than expected. These diseases include common debilitating diseases such as Alzheimer’s disease, Parkinson’s disease and other neurodegenerative disorders as well as epilepsies and childhood developmental diseases including forms of autism and learning disability.
“Our findings have shown that the human PSD is at centre stage of a large range of human diseases affecting many millions of people.”
Professor Seth Grant Wellcome Trust Sanger Institute
“Rather than ’rounding up the usual suspects’, we now have a comprehensive molecular playlist of 1000 suspects. Every seventh protein in this line-up is involved in a known clinical disorder, and over half of them are repeat offenders. Mining the postsynaptic proteome now gives researchers a strategic entry point, and the rest of us a front row seat to witness neuroscience unravel the complexity of human brain disorders.”
Professor Jeffrey L Noebels Professor of Neurology, Neuroscience and Human Genetics at Baylor College of Medicine
The findings open several new paths toward tackling these diseases.
“Since many different diseases involve the same set of proteins we might be able to develop new treatments that could be used on many diseases.”
Professor Seth Grant Sanger Institute
To aid in this objective the group has created the first molecular network, a roadmap of the molecular organisation of human synapses, which shows how the many proteins and diseases are interconnected.
“We also can see ways to develop new genetic diagnostic tests and ways to help doctors classify the brain diseases.”
Professor Seth Grant Sanger Institute
To accelerate discovery and application of their data, the scientists have released all their data into the public domain on their website – G2Cdb. The team suggests that the data on the proteome of the PSD will be extremely useful for understanding the brain in the same way the genome was useful for understanding DNA.
The scientists were able to use their study of diseases to identify the biological roots of human behaviour. They found that proteins in the PSD are especially important for cognitive behaviours such as learning and memory, emotion and mood, as well as social behaviours and addiction or drug abuse. The findings provide deep insights into how a DNA mutation can impact on fundamental aspects of our behaviour.
The team examined the rate of evolution of the PSD proteins over millions of years of mammalian evolution, expecting the proteins to evolve at the same rate as other proteins. In a fascinating and unexpected twist to the story, the team found that the PSD proteins changed much more slowly than expected, revealing that the PSD has been highly conserved or constrained from changing during evolution.
“The conservation of the structure of these proteins suggests that the behaviours governed by the PSD and the diseases associated with them have not changed much over many millions of years. It also shows that synapses in rodents are much more similar to humans than we expected showing that mice and rats are suitable models for studying human brain disease.”
Professor Seth GrantSanger Institute
This splendid collaborative study is a major step forward which will surely illuminate the causes of many of the major mental health and neurological disorders that are so common in Britain as well as indicating new ways to develop treatments for these most disabling diseases.”
Professor Jonathan R Seckl Moncrieff-Arnott Professor of Molecular Medicine and Executive Dean, College of Medicine and Veterinary Medicine, The Queen’s Medical Research Institute, Edinburgh
This study was made possible by a collaboration between three specialist teams including the neurosurgeons Professor Ian Whittle at Edinburgh University, the protein mass spectrometry facility led by Dr Jyoti Choudhary with Dr Mark Collins at the Sanger Institute and neuroscientists and computer biologists Dr Alex Bayés, Dr Louie Van de Lagemaat and Dr Mike Croning working in Professor Grant’s team. This project was conducted as part of the Genes to Cognition Program, which is a research program aimed at understanding the molecular basis of behaviour and brain disease.
More information
Funding
This work was supported by the Wellcome Trust, the Medical Research Council, the Scottish Higher Education Funding Council, the Melville Trust, the Marie Curie Foundation, the European Molecular Biology Organisation and the European Union.
Participating Centres
- Genes to Cognition Programme, Wellcome Trust Sanger Institute, Genome Campus, Hinxton, Cambridgeshire, UK
- Proteomic Mass Spectrometry, Wellcome Trust Sanger Institute, Hinxton, Cambridgeshire, UK
- Division of Clinical Neuroscience, Edinburgh University, Edinburgh, UK
Selected Websites
Publications:
Selected websites
Genes to Cognition Program
The Genes to Cognition Program (G2C) is an international collaborative program initiated by Professor Seth Grant and supported by the Wellcome Trust following the discovery that multiprotein complexes formed by intracellular proteins and neurotransmitter receptors were important for neuronal plasticity and behaviour.
Medical Research Council
For almost 100 years the Medical Research Council has improved the health of people in the UK and around the world by supporting the highest quality science. The MRC invests in world-class scientists. It has produced 29 Nobel Prize winners and sustains a flourishing environment for internationally recognised research. The MRC focuses on making an impact and provides the financial muscle and scientific expertise behind medical breakthroughs, including one of the first antibiotics penicillin, the structure of DNA and the lethal link between smoking and cancer. Today MRC funded scientists tackle research into the major health challenges of the 21st century.
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute, which receives the majority of its funding from the Wellcome Trust, was founded in 1992. The Institute is responsible for the completion of the sequence of approximately one-third of the human genome as well as genomes of model organisms and more than 90 pathogen genomes. In October 2006, new funding was awarded by the Wellcome Trust to exploit the wealth of genome data now available to answer important questions about health and disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.


