Brain Background to Body Mass
A genetic study of more than 90,000 people has identified six new genetic variants that are associated with increased Body Mass Index (BMI), the most commonly used measure of obesity. Five of the genes are known to be active in the brain, suggesting that many genetic variants implicated in obesity might affect behaviour, rather than the chemical processes of energy or fat metabolism.
Obesity is an increasing problem that results in individual risk to health as well as increasing burdens on health care systems. By identifying genetic variants that affect obesity, researchers hope to understand better the mechanisms regulating energy balance, which will guide the development of new therapies and help to develop improved diagnosis.
The study is published in Nature Genetics by the GIANT Consortium and includes authors from more than 60 institutions.
“It might seem remarkable that it is the brain that is most commonly influenced by genetic variation in obesity, rather than fat tissue or digestive processes. Until 2007, no genetic associations had been found for ‘common obesity’, but today almost all those we have uncovered are likely to influence brain function.”
Dr Inês Barroso A senior author on the study, from the Wellcome Trust Sanger Institute
Increase in weight occurs when calories taken in exceed calories burned, but behind that simple equation lie behavioural processes such as appetite and satiety, as well as the biochemical mechanisms our bodies use to process foods and use stores of energy, such as fat tissue. A part of the brain called the hypothalamus controls many of our basic functions such as body temperature, hunger and fluid balance: it is programmed to maintain the status quo.
“Very occasionally, mutations in genes active in the hypothalamus have dramatic consequences for weight gain such that people carrying these mutations are severely obese. Such mutations might be considered exceptional.
“However, we suggest that the picture for common obesity is very similar: many or most genes associated with increased BMI are active in the brain.”
Dr Ruth Loos A leading author from the Medical Research Council Epidemiology Unit
Studies in twins suggest that genetics can account for 40-70 per cent of the variation in BMI. Yet only one of the genes that were previously discovered had been thought to be linked to increased BMI or obesity in humans. The six new candidate genes provide a rich pool of resources to understand some of the processes in the brain that drive increased BMI and common obesity.
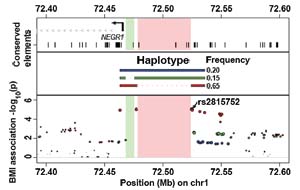
They also highlight the role of different types of mutation: intriguingly, one of the variants does not seem to mark a single-base change in the human genome, but rather the loss of a region of DNA of around 45,000 bases. The authors suggest that this lack of genome sequence near a gene called NEGR1might knock out genomic sequences that regulate activity of NEGR1. Such a variant has not been previously detected in studies of common obesity.
“It may seem surprising that we know so little about the biology of such an important medical and social issue. We can use genetics to open the door on some of the processes that contribute to individual differences in the predisposition to obesity. We are finding that common diseases have complex causes, and it is only by understanding the biology that we can start to make rational attempts to treat and prevent conditions such as this.
“Studies such as this are uncovering more and more genetic changes that are involved in more and more diseases. This is a remarkable time for human genetics.”
Mark McCarthy Robert Turner Professor of Diabetes at the University of Oxford, a senior author on the paper
The authors point out that perhaps dozens of similar variants remain to be discovered. The effects of the variants identified in the new study are modest: someone who carries all the risk variants would typically be 1.5-2 kg heavier than an average person. The findings of this study will pave the way for future investigations to uncover more of the elusive variants, perhaps through sequencing of whole genomes, which has become more efficient and cost-effective in the past year or so.
The research also helps to set the stage to unravel the two influences of genetics and environment. Future research could harness the power of longitudinal cohort studies, which track the health of many subjects through time, thereby providing the tools to map gene-environment interactions.
“As we uncover more variants, we will gain a better basic understanding of obesity, which in turn will open doors to previously unimagined areas of clinically relevant research. We hope that these advances will guide the development of more effective treatments and interventions.”
Joel Hirschhorn Associate Professor of Genetics at Children’s Hospital/Harvard Medical School and at the Broad Institute
More information
Funding
A full list of funding agencies is available at the Nature website.
Support for the Study
The authors are extremely grateful to all of the participants in each of the studies contributing to this effort. Full acknowledgments can be found in Supplementary Information.
Participating Centres
A full list of participating centres is available at the Nature website, which includes the GIANT (Genetic Investigation of ANthropometric Traits) consortium.
Publications:
Selected websites
Broad Institute
The Broad Institute of MIT and Harvard was founded in 2003 to bring the power of genomics to biomedicine. It pursues this mission by empowering creative scientists to construct new and robust tools for genomic medicine, to make them accessible to the global scientific community, and to apply them to the understanding and treatment of disease.
The Institute is a research collaboration that involves faculty, professional staff and students from throughout the MIT and Harvard academic and medical communities. It is jointly governed by the two universities.
Organized around Scientific Programs and Scientific Platforms, the unique structure of the Broad Institute enables scientists to collaborate on transformative projects across many scientific and medical disciplines.
Oxford University's Medical Sciences Division
Oxford University’s Medical Sciences Division is one of the largest biomedical research centres in Europe. It represents almost one-third of Oxford University’s income and expenditure, and two-thirds of its external research income. Oxford’s world-renowned global health programme is a leader in the fight against infectious diseases (such as malaria, HIV/AIDS, tuberculosis and avian flu) and other prevalent diseases (such as cancer, stroke, heart disease and diabetes). Key to its success is a long-standing network of dedicated Wellcome Trust-funded research units in Asia (Thailand, Laos and Vietnam) and Kenya, and work at the MRC Unit in The Gambia. Long-term studies of patients around the world are supported by basic science at Oxford and have led to many exciting developments, including potential vaccines for tuberculosis, malaria and HIV, which are in clinical trials.
Medical Research Council
The Medical Research Council supports the best scientific research to improve human health. Its work ranges from molecular level science to public health medicine and has led to pioneering discoveries in our understanding of the human body and the diseases which affect us all.
MRC Epidemiology Unit
The goal of the MRC Epidemiology Unit is to study the genetic, development and environmental determinants of obesity, diabetes and related metabolic disorders and to contribute to the scientific basis of prevention.
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute, which receives the majority of its funding from the Wellcome Trust, was founded in 1992. The Institute is responsible for the completion of the sequence of approximately one-third of the human genome as well as genomes of model organisms and more than 90 pathogen genomes. In October 2006, new funding was awarded by the Wellcome Trust to exploit the wealth of genome data now available to answer important questions about health and disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.


