Multi-drug resistant infection spreading globally among cystic fibrosis patients
A multi-drug resistant infection that can cause life-threatening illness in people with cystic fibrosis (CF) and can spread from patient to patient has spread globally and is becoming increasingly virulent, according to new research published today (10 November) in the journal Science.
The study, led by the Wellcome Trust Sanger Institute and the University of Cambridge, also suggests that conventional cleaning will not be sufficient to eliminate the pathogen, which can be transmitted through contaminated surfaces or in the air.
Mycobacterium abscessus, a species of multidrug resistant mycobacteria, has recently emerged as a significant global threat to individuals with cystic fibrosis and other lung diseases. It can cause a severe pneumonia leading to accelerated inflammatory damage to the lungs, and may prevent safe lung transplantation. It is also extremely difficult to treat – fewer than one in three cases is treated successfully.
It was previously thought that patients acquired the infection from the environment and that transmission between patients never occurred. The research team had previously studied one specialist CF centre in the UK and identified genetic and epidemiological evidence suggesting person-to-person transmission of M. abscessus but it was unclear whether this was a one off incident.
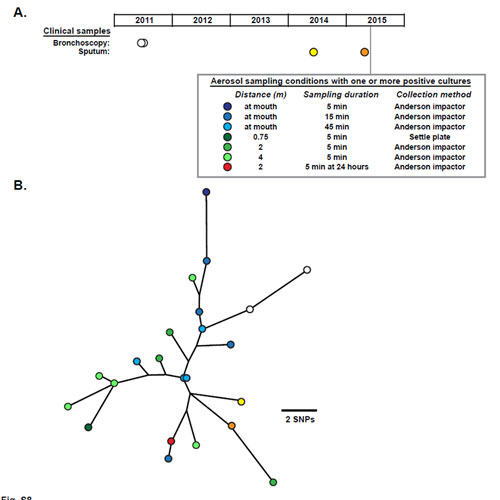
Now, by sequencing the whole genomes of over 1,000 isolates of mycobacteria from 517 individuals attending CF specialist centres in Europe, the US and Australia, researchers have demonstrated that the majority of CF patients have acquired transmissible forms of M. abscessus that have spread globally. Further analysis suggests that the infection may be transmitted within hospitals via contaminated surfaces and through airborne transmission. This presents a potentially serious challenge to infection control practices in hospitals.
Using a combination of cell-based and mouse models, the researchers showed that the recently-evolved mycobacteria were more virulent, likely to cause more serious disease in patients.
“This mycobacterium can cause very serious infections that are extremely challenging to treat, requiring combination treatment with multiple antibiotics for 18 months or longer. The bug initially seems to have entered the patient population from the environment, but we think it has recently evolved to become capable of jumping from patient to patient, getting more virulent as it does so.”
Professor Andres Floto from the Department of Medicine University of Cambridge, and the Cambridge Centre for Lung Infection at Papworth Hospital NHS Foundation Trust
“Our research should provide a degree of hope: now that we know the extent of the problem and are beginning to understand how the infection spreads, we can start to respond. Our work has already helped inform infection control policies and provides the means to monitor the effectiveness of these.”
Professor Julian Parkhill from the Wellcome Trust Sanger Institute at Hinxton Cambridgeshire
The Adult Cystic Fibrosis Centre at Papworth Hospital, Cambridgeshire, has led the development and implementation of new infection control policies to reduce the risk of transmission, now adopted across the UK and elsewhere. This study has also influenced the design of a new CF unit, due to open within the New Papworth Hospital on the Cambridge Biomedical Campus in 2018, which will incorporate a state-of-the-art air handling system.
“We uncovered clear evidence for transmission within clinics, and identified two possible transmission mechanisms, via fomites or aerosols, between patients. Using phylogenetic analysis we were able to unambiguously determine the spread of M. abscessus not only between individuals within specialist centres, but also from continent to continent.”
Josephine Bryant First author from the Sanger Institute
One question that the researchers will now aim to answer is how the pathogen manages to spread globally. The mechanism for this is unclear, but the researchers speculate that healthy individuals may be unwittingly carrying the mycobacteria between countries.
The sequencing data has also revealed potential new drug targets, and the team is now focused on working with other groups at the University of Cambridge and Colorado State University to develop these further.
“This paper highlights the risks posed through transmission of multi-drug resistant organisms between people with cystic fibrosis. The team in Cambridge are a world authority in this area. This work demonstrates the global threat of this infection, the risks of cross-infection within and between CF centres, and the need for improved surveillance. This study exemplifies the enormous impact of CF Trust-funded Strategic Research Centres, which were designed to generate world-class research with the very highest impact. Without the support of the CF community, this landmark study would not have been possible.”
Dr Janet Allen Director of Strategic Innovation at the UK Cystic Fibrosis Trust
Around one in 2,500 children in the UK is born with cystic fibrosis, a hereditary condition that causes the lungs to become clogged up with thick, sticky mucus. The condition tends to decrease life expectancy among patients.
More information
Funding
The research was funded by the Wellcome Trust and the UK Cystic Fibrosis Trust.
Publications:
Selected websites
About the University of Cambridge
The mission of the University of Cambridge is to contribute to society through the pursuit of education, learning and research at the highest international levels of excellence. To date, 96 affiliates of the University have won the Nobel Prize.
Founded in 1209, the University comprises 31 autonomous Colleges, which admit undergraduates and provide small-group tuition, and 150 departments, faculties and institutions.
Cambridge is a global university. Its 19,000 student body includes 3,700 international students from 120 countries. Cambridge researchers collaborate with colleagues worldwide, and the University has established larger-scale partnerships in Asia, Africa and America.
The University sits at the heart of one of the world’s largest technology clusters. The ‘Cambridge Phenomenon’ has created 1,500 hi-tech companies, 14 of them valued at over US$1 billion and two at over US$10 billion. Cambridge promotes the interface between academia and business, and has a global reputation for innovation. www.cam.ac.uk
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute is one of the world’s leading genome centres. Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease.
Wellcome
Wellcome exists to improve health for everyone by helping great ideas to thrive. We’re a global charitable foundation, both politically and financially independent. We support scientists and researchers, take on big problems, fuel imaginations and spark debate.
Cystic Fibrosis Trust
The Cystic Fibrosis Trust works towards a brighter future for everyone with cystic fibrosis by funding cutting-edge research, driving up standards of care and supporting people with the condition and their loved ones every step of the way.


