Protein controlling gut's protective force field identified
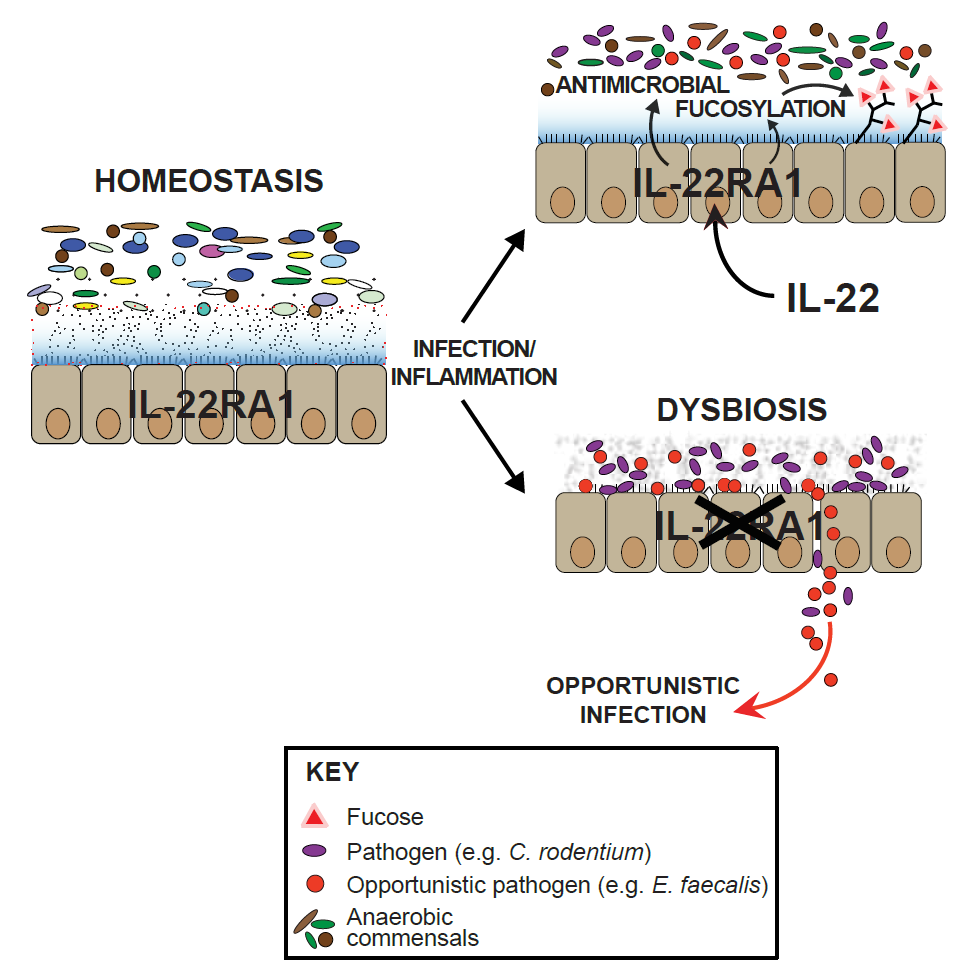
Scientists deleted the IL-22RA1 gene that produces the receptor protein from the mouse genome. In the absence of this gene, which is associated with inflammatory bowel disease (IBD) in humans, the mice were found to be more susceptible to over-colonisation by harmful Enterococcus faecalis bacteria.
“The gut prevents over-colonisation by fuelling the growth of bacteria that will restore balance. We confirmed this by treating the mouse models with the intestinal coating produced in wild-type mice and found that many of them regained their former equilibrium.”
Tu Anh Pham First author from the Wellcome Trust Sanger Institute
Investigating the mechanism further in the lab, researchers found that the protective force field works by inducing fucosylation, where a sugar-like substance is produced that coats the surface of the epithelial cells of the intestine, creating a healthy microbiota in which a whole host of protective bacteria will thrive.
Using organoids, an emerging research tool that enables scientists to grow small intestinal tissue clusters using cells from the original tissue and stem cells, researchers were able to take a closer look at epithelial cells in which IL-22RA1 receptors were working correctly.
Researchers sequenced the organoid RNA, the molecules that regulate gene expression in these cells, to identify the series of pathways affected by the activation of the IL-22RA1 receptor. Many of the pathways identified have previously been linked to autoimmune diseases such as IBD. In addition, Fut2, the gene involved in the related process of fucolysation, has known links to Crohn’s disease.
“In this research we’ve used the Sanger Institute’s expertise in mouse genetics to look at the microbiota in the context of a whole system and in extraordinary detail on a cellular level using organoids. Both perspectives are indispensable in our work to understand the complex interplay between the host and the unique mixture of bacteria each one of us harbours.”
Dr Trevor Lawley Faculty member and senior author from the Sanger Institute
Now that researchers have a better understanding of the host’s genetics, they can begin to identify the protective bacterial groups that proliferate when the IL-22RA1 receptor is activated. It is hoped that these bacterial species could be used therapeutically.
“We might, in the future, be able to harness what we know about this receptor and the bacteria it promotes to protect vulnerable patients. If we can replenish their microbiota and help them produce the correct environment in their gut, we will be able to give them the strength they need to battle infection.”
Professor Arthur Kaser Consultant Physician in the Department of Gastroenterology at Addenbrookes Hospital, Cambridge
More information
Funding
Please see the paper for details about funding.
Participating Centres
Please see the paper for a full list of participating centres.
Publications:
Selected websites
Mouse Genomes Project
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute is one of the world’s leading genome centres. Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.


