Clonal heterogeneity is hidden in the mix
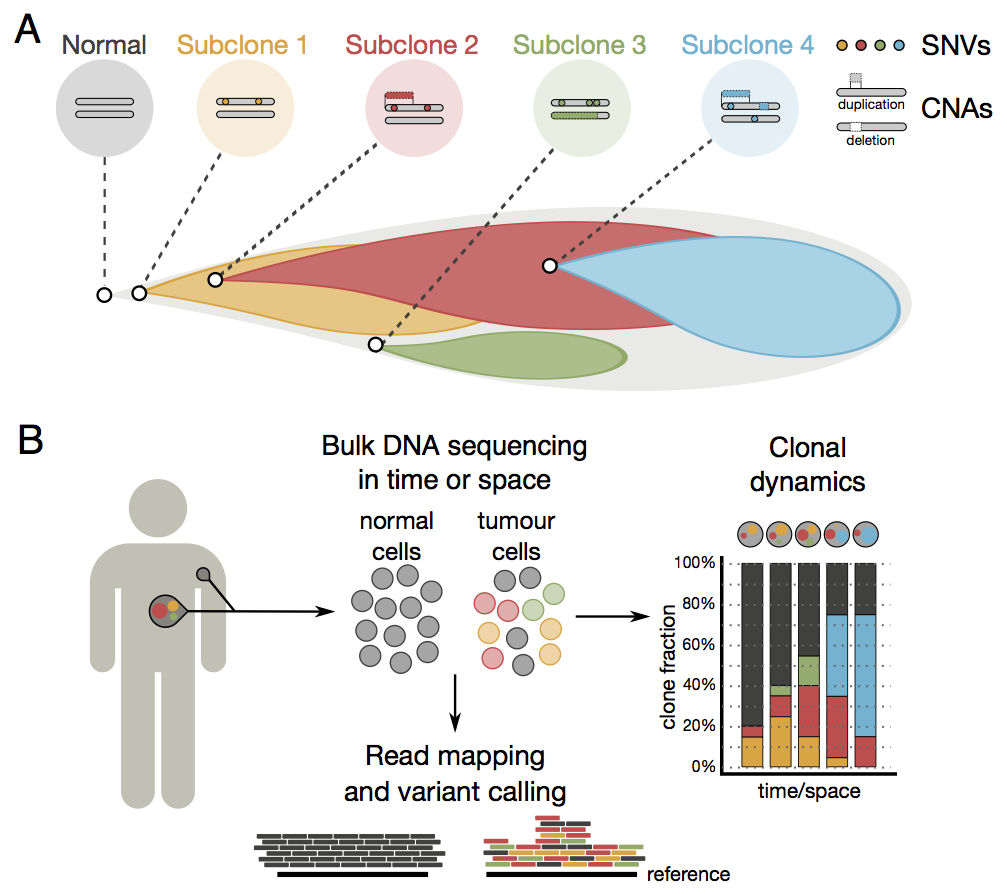
Cells in a tumour originate from a normal cell giving rise to billions of malignant, genetically diverse cells. Genome-wide approaches to study clonal heterogeneity are hampered by limited resolution of subpopulations, since short-read sequencing of mixed cell populations does not yield a direct readout of their clonal identity. There is mounting evidence for genetic heterogeneity in the majority of cancer types, which can translate in a differential response to treatment.
To address this problem, the algorithm uses short-read sequence data that can be related to different types of aberrations within all the genomes in a population to carry out its analysis. By combining these data the researchers can identify and describe the major lineages of genetically distinct cancer cells, also known as clones. The algorithm classifies cells into groups, discriminating different cell populations that belong to each of the clonal lineages. At the genetic level, the algorithm can assign aberrations by clone: somatic changes to single letters and copy-number changes in the genetic code, as well as allelic imbalances in the germline caused by these events.
“Our cloneHD algorithm helps us generate useful insights into cancer development and the effects of treatment, by delineating cell lineages within the tumour and their progression over time.”
Dr Ville Mustonen Senior author from the Sanger Institute
To test that the algorithm can trace clonal dynamics in cancer, the researchers retrospectively explored temporal changes in a case study of a patient with chronic lymphocytic leukaemia, from whom sequence data had been collected at five different time points during the course of treatment. Such retrospective analysis is helpful to better understand tumour evolution. They further performed a mimic of real-time monitoring by progressively adding more samples to see if prospective analyses are of potential clinical relevance.
“The algorithm allowed us to see that the dominant lineage of tumour cells diminished in response to treatment, but that another lineage of drug-resistant cells had started to rise in numbers. It is possible that, using this approach in conjunction with near real-time data collection, clinicians will be able to identify when a treatment is starting to fail.”
Dr Andrej Fischer First author from the Sanger Institute
In the near future, the team will test the algorithm and its potential clinical applications using larger data sets and a wider range of cancer types. Beyond cancer, a quantitative understanding of clonal dynamics translates to other organisms facing environmental stresses, such as bacteria, parasites or viruses.
More information
Funding
This work was funded by the Wellcome Trust, the German Research Foundation (DFG) and a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society.
Participating Centres
- Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, UK
- Department of Applied Mathematics and Theoretical Physics, Centre for Mathematical Sciences, University of Cambridge, Cambridge, UK
- Department of Genetics, University of Cambridge, Cambridge, UK
Publications:
Selected websites
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute is one of the world’s leading genome centres. Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.


