Harmless bacterium could be source of antibiotic resistance
Streptococcus pneumoniae is a bacterium that is a major global health problem. Although there are vaccines currently available against this bacterium, S. pneumoniae can evade the vaccine by exchanging its DNA in a process known as recombination. This can include the gain of antibiotic-resistant genetic variants and increase the risk of wider spread of antibiotic resistance.
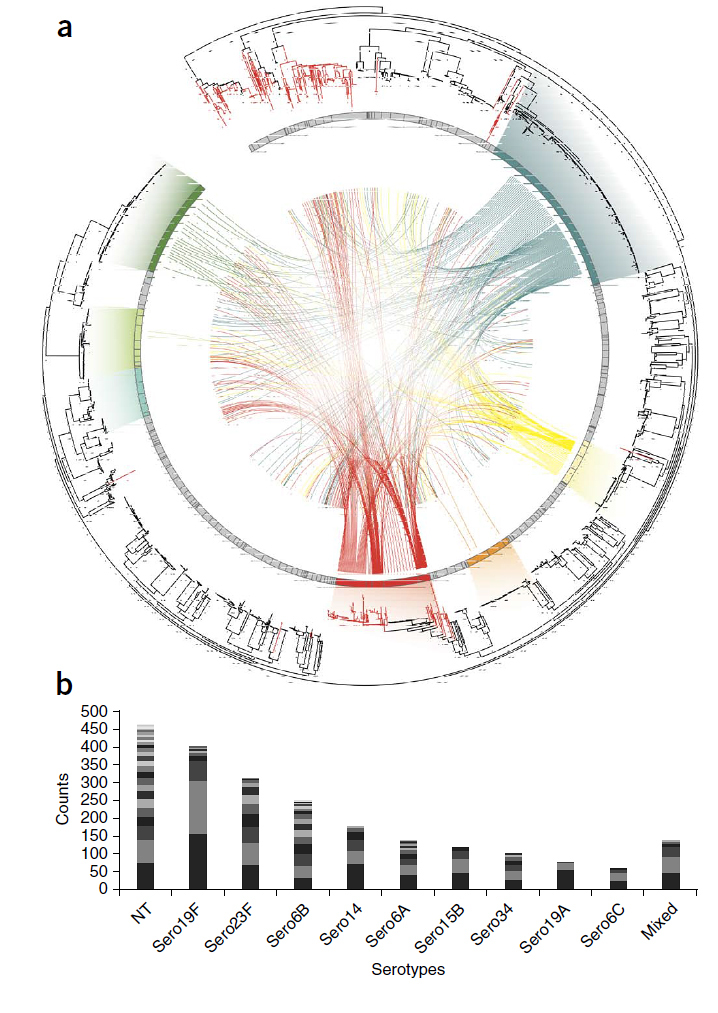
In this study, a seemingly harmless strain of the bacterium, known as non-typable or NT, was the most common type of S. pneumoniae found in over 3000 samples from individuals in a refugee camp. Although harmless, this strain is exchanging DNA at a faster rate than the other more harmful forms of the bacterium, including DNA carrying genes that carry antibiotic resistance.
“Vaccination programs have had a very positive impact on the rates of infection with resistant S. pneumoniae but we must remain vigilant as this pathogen is a master of adaptation with the potential to overcome our attempts at controlling it. This study shows that if we want to effectively deal with resistance, we have to keep track of this form of S. pneumoniae – we simply cannot afford to ignore it.”
Professor Stephen Bentley Senior author from the Wellcome Trust Sanger Institute
The team sequenced over 3000 samples of Streptococcus pneumoniae from almost 1000 infants and mothers from a refugee camp on the border between Myanmar and Thailand, to understand the evolution of the bacterium during healthy carriage and transmission from person to person. The proportion of people who carry S. pneumoniae tends to be greater in developing countries, as are the rates of pneumococcal disease.
They found that the most common form of this bacterium was one that rarely causes disease. These NT pneumococci lack the sugar capsule that generally coats all other disease-causing forms of the bacterium. However, the lack of a capsule means that the NT pneumococci can exchange DNA more readily than those enclosed in a sugar capsule.
The team found DNA exchange was exceptionally high in regions of the genome that are associated with antibiotic-resistance. They found that the swapping of antibiotic resistant regions of the genome has mediated the spread of resistance to the major family of ß-Lactam antibiotics which includes penicillin. This is consistent with the overuse of ß-Lactam antibiotics in the region since the 1990s.
“We can see with greater resolution, the process of recombination that provides an enhanced understanding of how this bacterium might acquire and lose antibiotic resistance. The reservoir of antibiotic-resistant genes is a real worry because it gives the pathogen population the potential to adapt to antibiotics more rapidly.”
Claire Chewapreecha First author from the Wellcome Trust Sanger Institute
Current vaccines target the sugar capsules that enclose these harmful bacteria. As the NT pneumococci does not have the sugar capsule, current vaccine cannot protect against the possibility of resistance genes being transferred from NT pneumococci.
“These NT pneumococci without the capsule are a real issue for us in the battle against antibiotic resistance. The two problems with NT pneumococci are firstly the prevalence of DNA exchange, especially antibiotic resistant genetic material, and secondly current vaccines cannot protect us against it – this is a dangerous combination. This research will help teams develop future intervention strategies.
“Our study would not have been possible without the help of the parents and children from Maela. We are incredibly grateful for their enthusiastic participation in the study.”
Dr Paul Turner Author from the Shoklo Malaria Research Unit
More information
Funding
This work was supported by grants from Wellcome Trust, Royal Thai Government, Academy of Finland, European Research Council, National Institute for Health Research, British Research Council.
Participating Centres
- Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, UK.
- Department of Infectious Disease Epidemiology, Imperial College London, St. Mary’s Hospital, London, UK.
- Shoklo Malaria Research Unit, Mahidol-Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Maesot, Thailand.
- Cambodia-Oxford Medical Research Unit, Angkor Hospital for Children, Siem Reap, Cambodia.
- Centre for Tropical Medicine, Nuffield Department of Medicine, University of Oxford, Oxford, UK.
- Helsinki Institute for Information Technology (HIIT), Department of Information and Computer Science, Aalto University, Finland.
- Department of Mathematics and Statistics, University of Helsinki, Helsinki, Finland.
- Immunobiology Unit, Institute of Child Health, University College London, London, UK.
- Department of Mathematics, Abo Akademi University, Turku, Finland.
- Department of Medicine, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK.
Publications:
Selected websites
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute is one of the world’s leading genome centres. Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.


