Immune system development linked to leukaemia
Scientists have discovered a genetic signature that implicates a key mechanism in the immune system as a driving force for a type of childhood leukaemia.
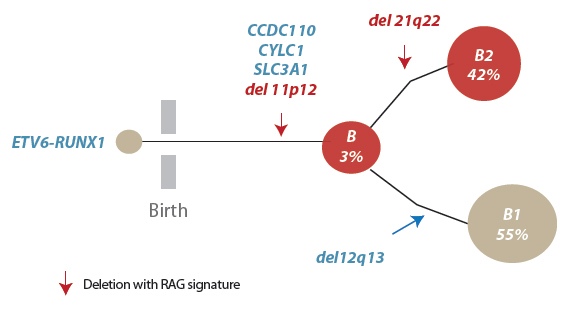
Acute lymphoblastic leukaemia or ALL is the most common form of childhood leukaemia. A key factor driving this leukaemia for one in four ALL patients is a mutation that causes two of their genes, ETV6 and RUNX1, to fuse together. This genomic alteration happens before birth and kick-starts the disease. But on its own the fusion gene cannot cause cancer; it requires additional mutations before the leukaemia can fully evolve and prompt symptoms.
The new study exploring how this process occurs was carried out by researchers from the Wellcome Trust Sanger Institute and The Institute of Cancer Research, London, with funding from the Kay Kendall Leukaemia Fund, Leukaemia and Lymphoma Research and the Wellcome Trust.
RAG proteins rearrange the genome in normal immune cells in order to generate antibody diversity. In ALL patients with the fusion gene, the team showed that these proteins can also rearrange the DNA of genes involved in cancer, leading to the development of leukaemia.
“For the first time, we see the combined events that are driving this treatable but highly devastating disease. We now have a better understanding of the natural history of this disease and the critical events from the initial acquisition of the fusion ETV6-RUNX1 to the sequential acquisition of RAG-mediated genome alterations that ultimately result in this childhood leukaemia.”
Dr Elli Papaemmanuil First author from the Wellcome Trust Sanger Institute
The team sequenced the genomes of 57 ALL patients with the fusion gene and found that genomic rearrangement and specifically deletions of DNA segments were the predominant drivers of the cancer. All samples showed evidence of events involving the RAG proteins.
The RAG proteins use a unique sequence of DNA letters as a signpost to direct them to antibody regions. The team found that remnants of this unique sequence lay close to more than 50 per cent of the cancer-driving genetic rearrangements. Importantly, this process often led to the loss of the very genes required for control of normal immune cell development.
It is the deletion of these genes that, in combination with the fusion gene, leads to the leukaemia. This striking genetic signature linking the RAG proteins to genomic instability is not found in other common cancers such as breast, pancreatic and prostate cancer, or other types of leukaemia.
“As we sequence more and more cancer genomes, we are increasingly understanding the mutational processes that underpin cancer’s evolution. In this childhood leukaemia, we see that the very process required to make normal antibodies is co-opted by the leukaemia cells to knock-out other genes with unprecedented specificity.”
Dr Peter Campbell Co-lead author from the Wellcome Trust Sanger Institute
To better understand the genetic events that led up to the development of this cancer, the team used single-cell genomics, a state-of-the-art technique that looks at the DNA from an individual cell. Using samples from two patients, they were able to show that this cancer-causing process occurs many times and results in a continuous diversification of the leukaemia.
“It may seem surprising that evolution should have provided a mechanism for diversifying antibodies that can collaterally damage genes that then contribute to cancer. But this only happens because the fusion gene (ETV6-RUNX1) that initiates the disease ‘traps’ cells in a normally very transient window of cell development where the RAG enzymes are active, teasing out their imperfect specificity.”
Professor Mel Greaves Co-senior author of the study from The Institute of Cancer Research, London
The team will now investigate how the RAG-mediated genomic instability accrues in cells with ETV6-RUNX1 fusion gene and what the role of this process is in the patients that relapse.
“The more we understand about the genetic events that underlie leukaemia and other cancers, the better equipped we are to develop improved diagnostics and targeted therapy for patients with this disease.”
Dr Peter Campbell
More information
Funding
This work was supported by the Kay Kendall Leukaemia Fund, the Leukemia and Lymphoma Research and the Wellcome Trust.
Participating Centres
- Cancer Genome Project, Wellcome Trust Sanger Institute, Hinxton, UK.
- Hospital Universitario 12 de Octubre, Madrid, Spain.
- Institute for Cancer Research, Sutton, London, UK.
- Department of Human Genetics, VIB and University of Leuven, Leuven, Belgium.
- Northern Institute for Cancer Research, University of Newcastle, Newcastle-upon-Tyne, UK.
- Centro Ricerca Tettamanti, Hospital San Gerardo, Monza, Italy.
- Department of Paediatric Haematology and Oncology, 2nd Faculty of Medicine, Charles University Prague and University Hospital Motol, Prague, Czech Republic.
- Paediatric Malignancy Unit, Molecular Haematology & Cancer Biology Unit, Camelia Botnar Laboratories, Great Ormond Street Hospital for Children and University College London (UCL) Institute of Child Health, London, UK.
- Department of Laboratory Medicine, University of California, San Francisco, San Francisco, California, USA.
- Addenbrooke’s National Health Service (NHS) Foundation Trust, Cambridge, UK. Department of Haematology, University of Cambridge, Cambridge, UK
Publications:
Selected websites
The Kay Kendall Leukaemia Fund
The Kay Kendall Leukaemia Fund was established in 1984 under the Will of James Sainsbury CBE. It awards grants for research on aspects of leukaemia and for relevant studies on related haematological malignancies. Grants are awarded for first class research on innovative proposals, particularly those close to the care of leukaemia patients or the prevention of leukaemia or related diseases. Project grants are awarded twice yearly, and Intermediate and Junior Fellowships of 3 – 4 years are awarded annually. The Fund also considers support for capital projects that will have direct benefit to leukaemia patient care.
Leukaemia & Lymphoma Research
Leukaemia & Lymphoma Research is the only UK charity dedicated to improving the lives of patients with all types of blood cancer, including leukaemia, lymphoma and myeloma. Its life-saving research is focused on finding causes, improving diagnosis and treatments, and running ground-breaking clinical trials for all blood cancer patients. Around 30,000 people of all ages, from children to adults, are diagnosed with blood cancers every year in the UK.
Institute of Cancer Research
The Institute of Cancer Research, London, is one of the world’s most influential cancer research institutes.
Scientists and clinicians at The Institute of Cancer Research (ICR) are working every day to make a real impact on cancer patients’ lives. Through its unique partnership with The Royal Marsden NHS Foundation Trust and ‘bench-to-bedside’ approach, the ICR is able to create and deliver results in a way that other institutions cannot. Together the two organisations are rated in the top four cancer centres globally.
The ICR has an outstanding record of achievement dating back more than 100 years. It provided the first convincing evidence that DNA damage is the basic cause of cancer, laying the foundation for the now universally accepted idea that cancer is a genetic disease. Today it leads the world at isolating cancer-related genes and discovering new targeted drugs for personalised cancer treatment.
As a college of the University of London, the ICR provides postgraduate higher education of international distinction. It has charitable status and relies on support from partner organisations, charities and the general public.
The ICR’s mission is to make the discoveries that defeat cancer.
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute is one of the world’s leading genome centres. Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.


