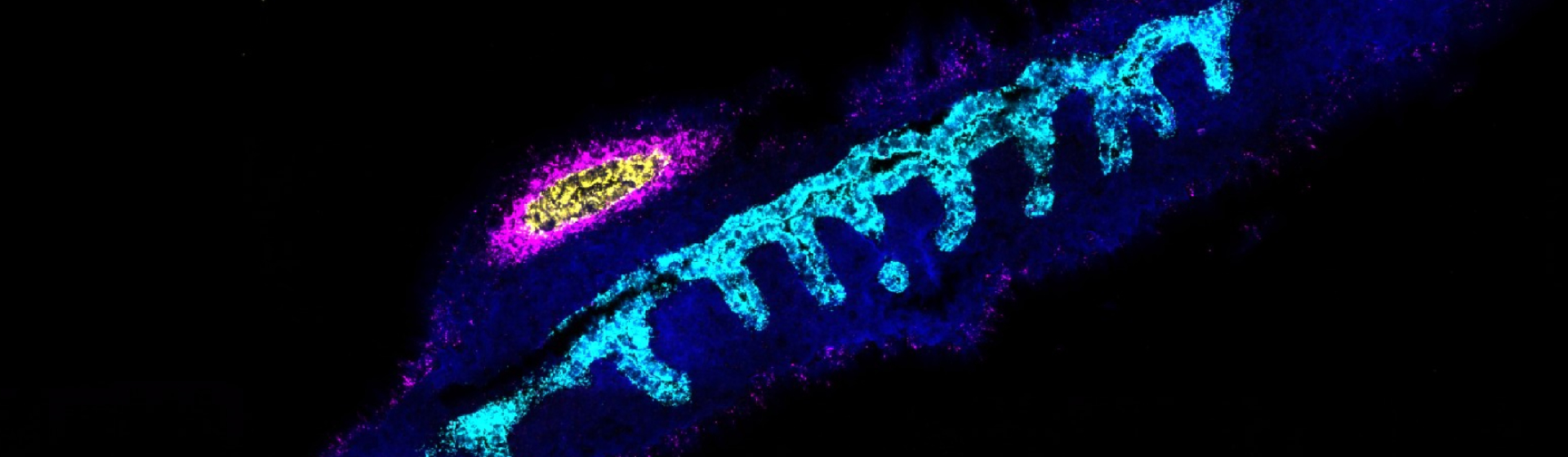
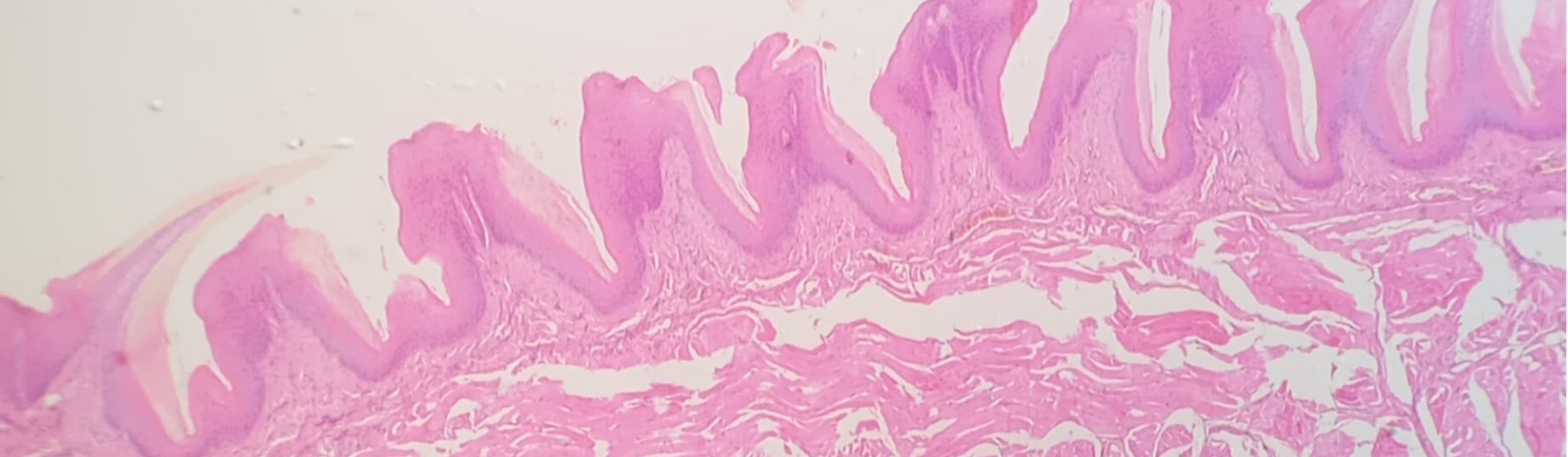
Stem cells could set up future transplant therapies
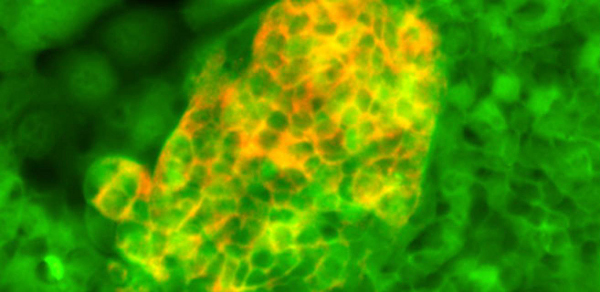
Using the technique, researchers have for the first time been able to grow a pure, self-renewing population of stem cells specific to the human foregut – the section of the digestive system that includes the liver and pancreas. These digestive cells could then be developed further to produce liver or pancreatic cells.
Stem cells have the potential to be used to replace dying or damaged cells with healthy cells and have potential wide-ranging uses in medicine such as organ replacement, bone replacement and treatment of neurodegenerative diseases.
This method significantly improves on existing techniques for cultivating these stem cells and raises the possibility that, with further work, they could be grown in large numbers. That would make it possible to use them for regenerative therapies, such as repairing damaged organs or tissues in the body.
“We have developed a cell culture system which allows us to specifically isolate foregut cells in the lab. These cells have huge implications for regenerative medicine, because they are the precursors to the thyroid, upper airways, lungs, liver, pancreas, stomach and biliary systems. We now have a system where we may be able to create all these cell types from the same starting population.”
Dr Nicholas Hannan From the University of Cambridge Wellcome Trust MRC Stem Cell Institute, Department for Surgery, who led the study, which was carried out in the lab of Dr Ludovic Vallier, joint Faculty member of the Wellcome Trust Sanger Institute and the University of Cambridge
To grow pancreatic or liver cells, stem cells are differentiated into the endoderm – the primary tissue layer associated with the digestive and respiratory systems. This provides a base population of cells that researchers can then try to develop as more specialised cells, such as heart or lung cells. Unfortunately, other cell types can grow, making it difficult to identify the target cells in the lab and complicating the application of these cells in transplant therapies.
The new approach overcomes some of these problems that currently limit scientists’ ability to grow cells associated with this region in sufficiently large numbers for clinical use.
By manipulating the signal pathways of the cells and varying the environment in which the cells were developed, researchers were able to isolate the precise treatments needed to drive differentiation of cells associated with the foregut itself. When heavily contaminated stem cell populations were developed under these conditions, the contaminating cells eventually died out.
The universal nature of this culture system takes a step towards a universal system that could be used to treat any patient requiring cells for transplantation purposes.
The cells generated are true stem cells because they are able to self-renew and can differentiate towards any part of the foregut. Because they are also still at the stage where they can self-renew, they can be grown in large enough numbers to be used in clinical therapies. The team was also able to show that these human foregut stem cells do not form tumours, which means that they could be safely injected for therapeutic purposes, without having adverse side effects.
“What we have now is a better starting point – a sustainable platform for producing liver and pancreatic cells. It will improve the quality of the cells that we produce and it will allow us to produce the large number of uncontaminated cells we need for the clinical application of stem cell therapy.”
Dr Ludovic Vallier Senior author of the study
More information
Funding
This work was funded by a MRC senior nonclinical fellowship, the Cambridge Hospitals National Institute for Health Research Biomedical Research Centre, the EU grant LivES, the Evelyn trust, the Medical Research Council, and the Wellcome Trust.
Participating Centres
- Wellcome Trust-Medical Research Council Stem Cell Institute, Anne McLaren Laboratory for Regenerative Medicine, Department of Surgery, West Forvie Site, Robinson Way, University of Cambridge, Cambridge CB2 0SZ, UK
- Wellcome Trust-Medical Research Council Stem Cell Institute, Tennis Court Road, Cambridge CB2 1QR, UK
- Wellcome Trust Sanger Institute, Hinxton CB10 1SA, UK
- Centre for Endocrinology and Diabetes, Institute of Human Development, Faculty of Medical and Human Sciences, Manchester Academic Health Sciences Centre, University of Manchester, Oxford Road, Manchester M13 9PT, UK
Publications:
Selected websites
The Wellcome Trust-Medical Research Council Stem Cell Institute
The Wellcome Trust-Medical Research Council Stem Cell Institute explores and defines the properties of stem cells to establish their true medical potential. Leading research scientists, technology specialists and doctors work side by side to create a world-leading centre of excellence in stem cell biology and medicine. The Institute also provides high level training for young researchers from around the world and collaborates with bio-industry.
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute is one of the world’s leading genome centres. Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.