Resetting genetic instructions
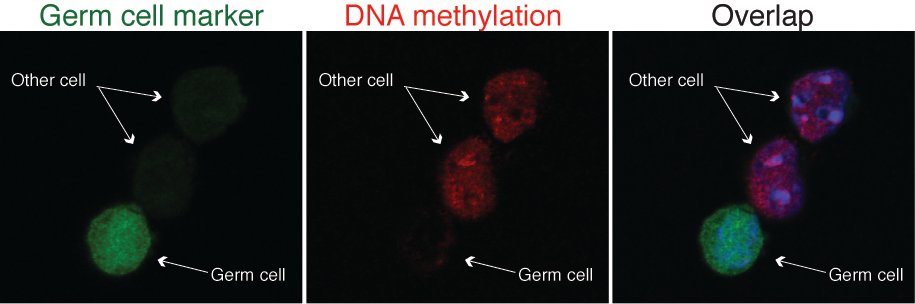
Researchers have gained a much clearer view of how sperm and egg cells are genetically reset in order that they function properly during fertilization and development. The new study, published on 6 December 2012 in Molecular Cell, is the first genome-wide study to look at what happens to chemical tags that affect DNA activity during early stages of egg and sperm cell development.
The team show that the process of resetting, in which chemical tags in the parent cells are erased from the genome, occurs earlier in the development of sperm and egg than thought. They also show, crucially, that some regions of the genome escape the widespread erasure, carrying their tags to the next generation.
All the cells in the body of one individual share the same DNA sequence: the way in which the DNA sequence is interpreted by each cell – whether genes are switched on or off, for example – results in the formation of different cell types. Genes that are inactive often acquire a chemical tag, called a methyl group, that plays a role in controlling gene activity.
Such potentially reversible changes to genes can occur during the life of a cell and of an organism, and can be influenced by cues from outside. This type of research, investigating modifications to the DNA which do not alter the underlying DNA sequence, is called epigenetics.
The team examined the timing and extent of changes to the pattern of methyl groups in primordial germ cells – cells that are destined to become egg and sperm in the adult animal.
“We produced a high-resolution map showing the location and timing of methyl group removal from primordial germ cell DNA. We discovered that the majority of demethylation occurred much earlier than people previously thought and this has allowed us to shed light on the process of methyl group removal in mammals, a mechanism that has remained elusive for many years.
“An even more exciting finding is that we have identified regions of DNA that avoid demethylation and are therefore candidates for how environmental information can be transferred from parent to offspring. Interestingly, one of these regions is linked to the development of type 2 diabetes.”
Dr Stefanie Seisenberger Lead author from the Babraham Institute, which receives strategic funding from the Biotechnology and Biological Sciences Research Council (BBSRC)
“Several recent studies in other laboratories have confirmed that environmental information can be transferred from parent to offspring in mammals, for example mice fed a high-fat diet produce offspring with altered metabolic regulation, but it is not known how this occurs. One interesting observation from our study, which backs up work performed elsewhere, is that incomplete removal of methyl groups from DNA occurs more frequently in sperm than egg forming cells, suggesting that fathers have a bigger part to play in epigenetic inheritance than previously thought. This has implications not only for understanding mechanisms of inheritance and development but also our susceptibility to obesity and diseases like diabetes.”
Professor Wolf Reik Senior author of the paper, a Group Leader at the Babraham Institute and an associate faculty member at the Wellcome Trust Sanger Institute
Part of the work performed in this study was carried out at the Wellcome Trust Sanger Institute under the new Associate Faculty scheme.
“This is an excellent example of fruitful scientific collaboration between research institutes bringing about an improvement of our knowledge of how we develop, and thus paving the way for future epigenetics research into the inheritance of age-related diseases such as diabetes.”
Professor Michael Wakelam Director of the Babraham Institute
This research was supported by the BBSRC, Boehringer Ingelheim Fonds, MRC, Wellcome Trust and the EU.
More information
Funding
This research was supported by the BBSRC, Boehringer Ingelheim Fonds, MRC, Wellcome Trust and the EU.
Publications:
Selected websites
The Babraham Institute
The Babraham Institute, which receives strategic funding (£22.4M in 2010-11) from the Biotechnology and Biological Sciences Research Council (BBSRC), undertakes international quality life sciences research to generate new knowledge of biological mechanisms underpinning ageing, development and the maintenance of health. The Institute’s research is focused on understanding the biological events that underlie the normal functions of cells and the implication of failure or abnormalities in these processes. Research focuses on signalling and genome regulation, particularly the interplay between the two and how epigenetic signals can influence important physiological adaptations during the lifespan of an organism. By determining how the body reacts to dietary and environmental stimuli and manages microbial and viral interactions, we aim to improve wellbeing and healthier ageing.
BBSRC invests in world-class bioscience research and training on behalf of the UK public. Our aim is to further scientific knowledge, to promote economic growth, wealth and job creation and to improve quality of life in the UK and beyond. Funded by Government, and with an annual budget of around £445M, we support research and training in universities and strategically funded institutes. BBSRC research and the people we fund are helping society to meet major challenges, including food security, green energy and healthier, longer lives. Our investments underpin important UK economic sectors, such as farming, food, industrial biotechnology and pharmaceuticals.
Websites
- www.babraham.ac.uk
- For more information about BBSRC, our science and our impact see: www.bbsrc.ac.uk
- For more information about BBSRC strategically funded institutes see: www.bbsrc.ac.uk/institutes
The Boehringer Ingelheim Fonds
The Boehringer Ingelheim Fonds (BIF) is a public foundation – an independent, non-profit organization for the exclusive and direct promotion of basic research in biomedicine. The foundation supports up-and-coming scientists whose research projects experimentally elucidate the basic phenomena of human life. BIF also organizes two International Titisee Conferences per year. At BIF, we are convinced that great scientific ideas and developments need a stimulating context, freedom and a sound financial fundament. We do our best to provide all this for our fellows in our various fellowship and grant programmes.
Medical Research Council
For almost 100 years the Medical Research Council has improved the health of people in the UK and around the world by supporting the highest quality science. The MRC invests in world-class scientists. It has produced 29 Nobel Prize winners and sustains a flourishing environment for internationally recognised research. The MRC focuses on making an impact and provides the financial muscle and scientific expertise behind medical breakthroughs, including one of the first antibiotics penicillin, the structure of DNA and the lethal link between smoking and cancer. Today MRC funded scientists tackle research into the major health challenges of the 21st century.
EpiGeneSys
As an FP7 European Community-funded Network of Excellence, EpiGeneSys’s goals go further than simply funding a research project-our extensive training program is helping to build a bridge between the fields of epigenetics and systems biology and our public education mission will communicate the science in an accessible and interesting fashion while awakening young pupils’ interest in research.
EU BLUEPRINT
EU BLUEPRINT is a large-scale research project receiving close to 30 million euro funding from the EU. 41 leading European universities, research institutes and industry entrepreneurs participate in what is one of the two first so-called high impact research initiatives to receive funding from the EU.
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute is one of the world’s leading genome centres. Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.


