Biology behind brain development disorder
By using a combination of research in mice and sequencing the DNA of four patients with the disorder, the team showed that disruption of this gene causes symptoms including brain abnormalities and reduced growth, highlighting the power of mouse models for understanding the biology behind rare diseases.
“Ubiquitination, the biological pathway UBE3B is involved in, is crucial in neurodevelopment. We have studied several patients with this rare condition, and by sequencing the coding regions of the genome of these patients we found mutations implicating the gene UBE3B. This result was confirmed by studies performed in mice by our collaborators at the Sanger Institute.”
Dr Guntram Borck Lead author from the University of Ulm
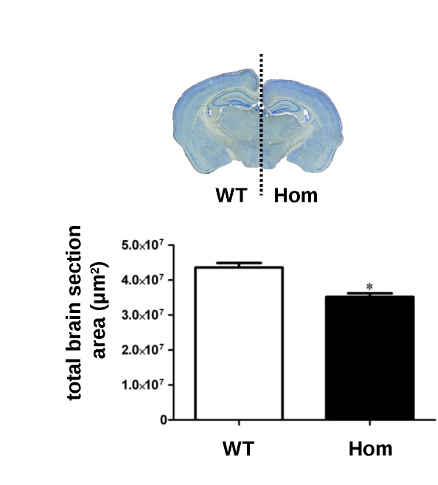
At the Sanger Institute researchers deleted the gene in mice and found that they had symptoms that were quite similar to those in the patients with UBE3B mutations including; reduced body weight and size, and reduced size of the brain.
The studies in mice also uncovered other defects underlying the disorder. Mice with the gene deletion had reduced cholesterol levels, a symptom that was seen by the team in three of the patients. This observation suggests that a defect in cholesterol metabolism is associated with this syndrome.
“Both techniques, DNA sequencing and deleting the gene in mice, support the finding that disruption of UBE3B causes this syndrome. We can now learn much more about this syndrome by studying these mice. They also represent a pre-clinical model in which we may trial potential new therapies.”
“This is the first time that this gene has been implicated in any disorder.”
Dr David Adams Lead author from the Wellcome Trust Sanger Institute
DNA sequencing has greatly improved the identification of variants associated with developmental disorders. But the challenge still remains for researchers to identify which of these variants, there are usually several hundred identified in each patient, cause the disorder. Animal models are a complementary approach for determining the causal gene and for understanding the biology behind genetic disorders.
“The syndromes we are studying, although rare, tell us a huge amount about biology. Understanding the biology behind these disorders could improve our management of these rare conditions and provide new avenues of research into treatments for scientists to pursue.”
Dr Helen Firth Consultant Clinical Geneticist at Cambridge Universities Hospital Trust
More information
Funding
This work was supported by the Deutsche Forschungsgemeinschaft, the Rubicon European Union Network of Excellence, the Israeli Ministry of Health Chief Scientist Foundation and the Israeli Science Foundation, the Wellcome Trust, the MRC and the EC.
Participating Centres
A full list of participating centres can be found in the paper.
Publications:
Selected websites
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute is one of the world’s leading genome centres. Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.


