Preventing the devil's downfall
Researchers have sequenced the genome of a contagious cancer that is threatening the Tasmanian devil, the world’s largest carnivorous marsupial, with extinction. Cataloguing the mutations present in the cancer has led to clues about where the cancer came from and how it became contagious.
The research has revealed that the cancer, which is spread between animals by biting, first arose from the cells of a single female Tasmanian devil. This animal is nicknamed ‘The Immortal Devil’ because although she died more than 15 years ago, her DNA is living on in the contagious cancer cell line that she spawned. The cancer causes the appearance of tumours on the face of affected devils which grow rapidly and cause death within months.
“The Tasmanian devil cancer is the only cancer that is threatening an entire species with extinction. Sequencing the genome of this cancer has allowed us to catalogue the mutations that caused this cancer to arise and to persist in the Tasmanian devil population.”
Dr Elizabeth Murchison Lead author from the Wellcome Trust Sanger Institute
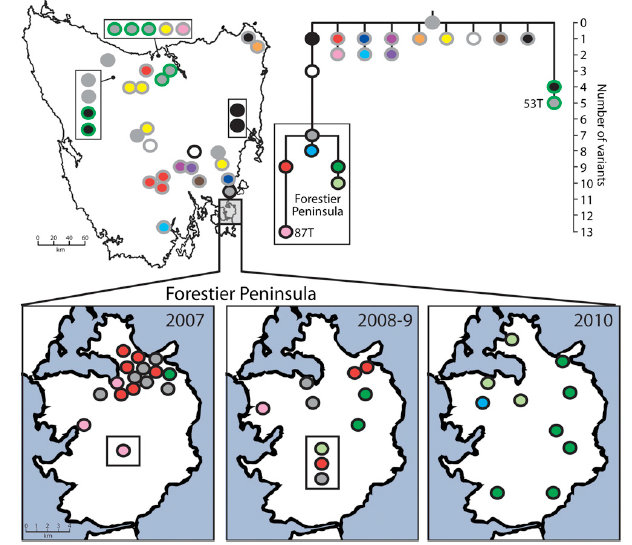
“We found that devil cancer’s genome has about 20,000 mutations. This is fewer mutations than are found in some human cancers and indicates that cancers do not need to be extremely unstable in order to become contagious. Tracing the evolutionary history and spread of this cancer helps us to understand not only what caused this disease but also to predict how it might behave in the future.”
Dr David Bentley Senior co-author from Illumina Cambridge Ltd
The spread of cancer between individuals is normally prevented by the immune system, which can normally detect foreign tissues as ‘non-self’. The team found some intriguing clues as to how the devil cancer may outwit the immune system, including mutations in a set of genes involved in immunity. However, future studies will be required to elucidate how cancer escapes the immune destruction.
“This research is important because it allows us to understand the pattern of disease spread and this may help contain the epidemic. However, we also now need to use the genome sequence to understand more about how this cancer became transmissible. Cancers that transmit through populations are obviously incredibly rare, but we should use the Tasmanian devil example to understand the process to be prepared in the extremely unlikely event that such an epidemic ever occurs in humans.”
Professor Mike Stratton Senior author and Director of the Wellcome Trust Sanger Institute
The next stage of the research will be to map the genomes of thousands of devil tumours in order to understand the genetic diversity present in the cancer and to investigate the genetic interactions between the cancer and the Tasmanian devil population.
More information
Funding
This work was supported in part by a Wellcome Trust, Dr Eric Guiler Tasmanian Devil Research and a L’Oreal UNESCO For Women in Science Fellowship, UK and Ireland (EPM).
Participating Centres
A list of participating centres can be found on the paper.
Publications:
Selected websites
Illumina
Illumina is a leading developer, manufacturer, and marketer of life science tools and integrated systems for large-scale analysis of genetic variation and function. We provide innovative sequencing and array-based solutions for genotyping, copy number variation analysis, methylation studies, gene expression profiling, and low-multiplex analysis of DNA, RNA and protein. We also provide tools and services that are fueling advances in consumer genomics and diagnostics. Our technology and products accelerate genetic analysis research and its application, paving the way for molecular medicine and ultimately transforming healthcare.
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute is one of the world’s leading genome centres. Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.


