Zebrafish provide insight into congenital muscular dystrophies
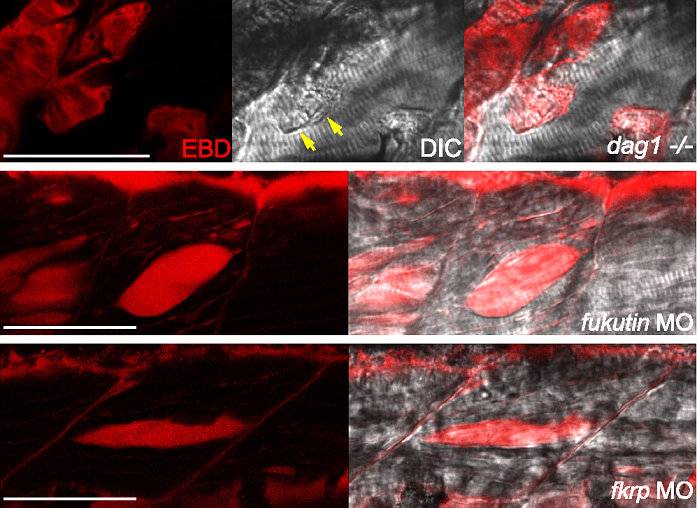
Researchers have unmasked biological processes that may lead to a range of muscular dystrophies commonly known as dystroglycanopathies. The team used zebrafish to recapitulate various molecular and clinical aspects of the disease. Dystroglycanopathies are inherited neuromuscular disorders, the most severe forms of which affect young children.
The team suggests that perturbations to the way protein is packed in the cell and the cell’s stress response when this process goes wrong could be critical in the biology of the disease and might explain why some patients experience more severe symptoms than others.
Muscular dystrophies are a heterogenous group of muscle diseases, characterised by muscle degeneration and weakness: most develop gradually over the course of a lifetime. However, approximately one in 20,000 to 50,000 babies in the UK is born with congenital muscular dystrophies. Children born with congenital muscular dystrophies typically start to show symptoms at birth or in the early months of their lives. They often are unable to walk. Currently, there is no effective treatment.
The study looks at a group of congenital muscular dystrophies, called dystroglycanophathies, which are associated with reduced sugar modification on a protein called dystroglycan. Symptoms include muscle wasting and, in the most severe cases, structural abnormalities of the eyes and brain, with learning difficulties.
“Understanding precisely what mechanism leads to muscle damage and brain and eye involvement in patients with this kind of muscular dystrophy is a difficult task. Muscle biopsies taken from patients with dystroglycanopathies have consistently shown that they have abnormalities in the sugar modification of dystroglycan. Previous studies have also found several genes that seem to affect this process. One of these genes, fukutin-related protein (fkrp), is responsible for the most common dystroglycanopathy variant in UK.
“However the precise function of this gene, and its related fukutin gene, remains unclear.”
Francesco Muntoni from the Dubowitz Neuromuscular Centre, Institute of Child Health, University College London, and an author on the paper
Researchers had previously suggested that Fukutin and FKRP might be involved in a critical step during the packing of dystroglycan protein – adding sugar to the protein. This step transforms the protein into a functional receptor, which can interact with binding partners outside of the cell.
The team looked at ways to understand the molecular and cellular mechanisms leading to Fukutin- or FKRP-deficient dystroglycanopathies. To do this, they used zebrafish to study the function of Fukutin and FKRP in relation to dystroglycan in more detail.
“Zebrafish embryos develop outside of the mother’s body from the moment of fertilisation. This makes them an ideal system to study congenital diseases, especially when the disease may cause lethality or severe defects before birth. By disabling, in turn or in combination, the zebrafish Fukutin, FKRP and dystroglycan proteins, we could compare, in real time, the effects on muscle integrity and function.
“If disrupting Fukutin or FKRP was indeed disabling dystroglycan by stopping the addition of sugar to the protein, we would expect to see the same effect irrespective of whether we turn off the fukutin and fkrp genes or remove the dystroglycan protein itself. In practice, what we saw was startlingly different.”
Yung-Yao Lin from the Wellcome Trust Sanger Institute and first author on the paper
One of the most striking differences the team observed was the impact on the body length of the fish. When the team disabled the fukutin and fkrp genes, they saw stunted developmental defects and shortened fish; loss of dystroglycan, however, had no effect on the length of the fish. In addition, the manifestation of muscle defects in embryos lacking Fukutin or FKRP was different from that of zebrafish with a complete absence of dystroglycan. Finally, the team showed that several molecular markers also distinguish embryos lacking Fukutin or FKRP from embryos lacking dystroglycan.
Together, the results suggest that Fukutin and FKRP seem to do more than affect sugar modification of dystroglycan alone.
“The theory that Fukutin and FKRP’s role in muscular dystrophies is simply due to their ability to add sugar to dystroglycan protein seems to underestimate the role of these genes. What we report for the first time in this study, is that disabling Fukutin or FKRP seems to perturb the very machinery of the cell’s protein packing factory where dystroglyan and other secreted proteins are modified. When these genes are missing, the production line fails and we see an accumulation of unpacked or partly packed proteins in the cell – a stress condition for the cell.
“In response to this accumulation, the cell must choose a strategy to ease the stress. Our research suggests that this ‘stress response’ may be a crucial factor underlying Fukutin- and FKRP-deficient dystroglycanopathies in humans.”
Derek Stemple from the Sanger Institute and senior author on the paper
The cell’s stress response can vary from damage limitation measures to, in the worst case, self-destruction. The researchers suggest that the strategy the cell adopts could determine the severity of symptoms and could go some way towards explaining why some patients with dystroglycanopathy suffer muscle weakness alone, while others have more severe symptoms affecting the brain and learning.
The research is a critical step towards understanding the way genetic changes give rise to the symptoms seen in patients and might inform the development of therapeutic strategies in the future. The team suggests that further studies will be needed to fully understand the biological role of Fukutin and FKRP in muscular dystrophies and look at ways of managing stress response when protein packing goes wrong.
More information
Funding
This work was supported by the Wellcome Trust and the ZF-MODELS Integrated Project, funded by the European Commission.
Participating Centres
- Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, CB10 1SA, United Kingdom
- Dubowitz Neuromuscular Centre, Institute of Child Health, University College London, London United Kingdom
Publications:
Selected websites
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute, which receives the majority of its funding from the Wellcome Trust, was founded in 1992. The Institute is responsible for the completion of the sequence of approximately one-third of the human genome as well as genomes of model organisms and more than 90 pathogen genomes. In October 2006, new funding was awarded by the Wellcome Trust to exploit the wealth of genome data now available to answer important questions about health and disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.


