Forces for cancer spread: genomic instability and evolutionary selection
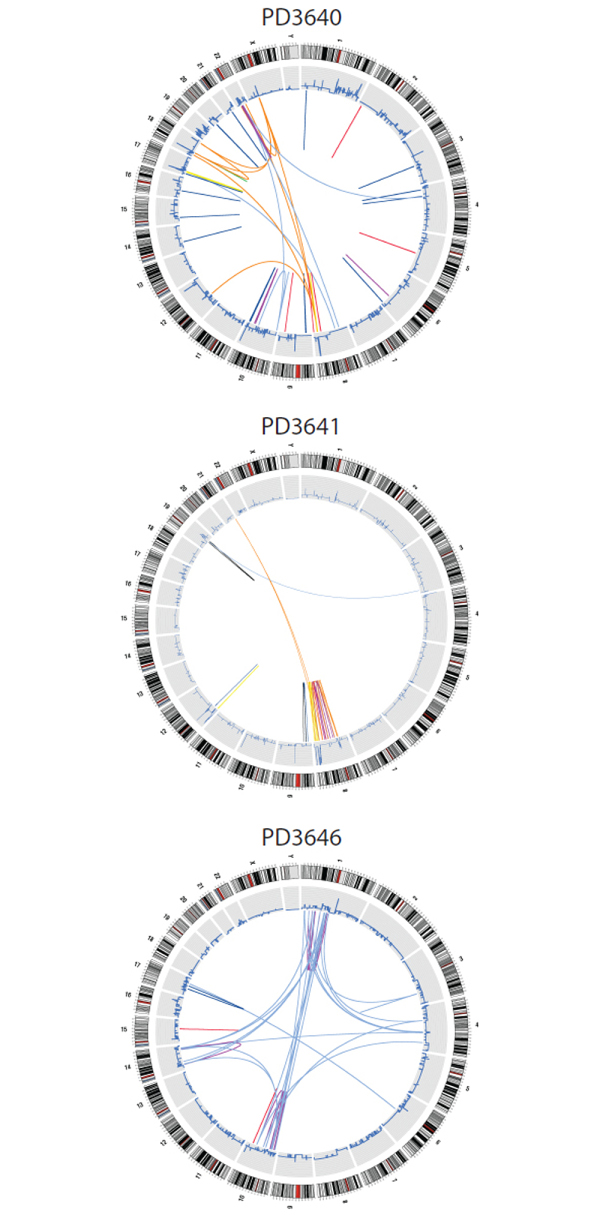
In new research published today, researchers uncover evolution in action in cancer cells. They show the forces of evolution in pancreatic tumours mean that not only is cancer genetically different between different patients, but each new focus of cancer spread within a patient has acquired distinct mutations.
Effectively, 10 different foci of cancer spread are ten different, but related, tumours. The complexity of pancreatic cancer genetics uncovered in this work helps to explain the difficulty of treating the disease but also strengthens the need for improved methods for early diagnosis
Pancreatic cancer is an aggressive malignancy with only two or three patients in one hundred living beyond five years from first diagnosis. The spread – metastasis – of the tumour is thought to be relatively symptomless in most patients until the disease is advanced.
“We have always known that pancreatic cancer is a particularly aggressive disease. This study illustrates why it is so challenging. Each metastasis is its own tumour, each evolving, each striving for dominance, each adapting to life outside the pancreas. When we treat cancer that has spread through the body, we’re not just treating one tumour, we might be treating tens of genetically distinct tumours.”
Dr Peter Campbell from the Wellcome Trust Sanger Institute and first author on the paper
The researchers, from the Wellcome Trust Sanger Institute, near Cambridge, UK and the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins Medical Institutions, Baltimore, USA, looked at cancers in 13 patients who died from pancreatic cancer. They mapped rearrangements in the genomes of cancer samples: in some cases, they looked at several metastases from a patient.
They discovered that pancreatic cancer genomes often contain a distinctive pattern of genome rearrangement that possibly reflects changes to repair mechanisms in the cancer cells. The pattern of mutation events is dramatically different to that found in breast cancer, for example.
“With each study, cancer genomes are being revealed in their intricate complex detail. Genome instability is common in cancer, but this study has further revealed the dynamic nature of that instability and its role in spread of disease in the patient – with instability being an engine of selection that allows the tumour to adapt to new sites in the body.
“We can see a root of common lesions – about half of the mutations are shared across metastases. Metastatic cancer is therefore like a family: the different deposits of tumour are genetically related to one another, as brothers, sisters and cousins are, but also have distinguishing genetic features that make them individual. Identifying and targeting the shared mutations with drugs is likely to be a route to more effective treatment.”
Dr Andy Futreal Head of Cancer Genetics and Genomics at the Wellcome Trust Sanger Institute and a senior author on the paper
In a companion study Dr Iacobuzio-Donahue and her colleagues show that single-letter mutations show a similarly complex pattern. The team on that paper suggest that there might be a long time lag from the first cancer-causing mutations in the primary tumour to the violent and rapid metastasis of late-stage disease.
Both papers suggest that the galloping mutation rate that develops produces cells that, because of specific mutations they acquire, can colonize other organs. Different combinations of active genes are needed to survive in different tissues. This is a return to the 120-year-old seed and soil hypothesis that some organs provide particularly fertile ground for particular cancer cells to grow. This work shows that even in one person’s cancer, clones of cells can evolve genomes specialised for life in defined organs.
The researchers emphasize that the shared mutations common to many early-stage pancreatic cancers could provide a route to discovery of new drug targets. In addition, the long time between the initial genetic changes in the developing primary cancer and spread to other organs might offer a window in which early diagnosis could detect disease while it is still curable by surgery.
The patients for these studies were recruited to a programme established by Dr Iacobuzio-Donahue in Baltimore to develop new understanding of this difficult tumour type. Patients with terminal pancreatic cancer discuss with the team the aims of the research and choose whether or not to provide samples after their death.
“We are so grateful to all patients who have discussed this programme. In times of tremendous personal difficulty, they and their families and friends have taken steps to help others, with the hope we can improve diagnosis and treatments in the future. The sacrifices they have made are now fundamentally improving our understanding of how pancreatic cancer develops and spreads.
“This is a research paper, but we are all of us aware that there are real people behind these samples.”
Dr Iacobuzio-Donahue from the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins Medical Institutions Baltimore, Maryland and a senior author on the paper
More information
Publication details
- The patterns and dynamics of genomic instability in metastatic pancreatic cancer.
Campbell PJ, Yachida S et al.
Nature 2010
Published online on 28.10.10 prior to print production.
DOI: 10.1038/nature09460
The other publication referred to in this release is:
- Distant metastasis occurs late during the genetic evolution of pancreatic cancer.
Yachida S et al.
Nature 2010
Published online on 28.10.10 prior to print production.
Funding
This work was supported by the Wellcome Trust, the Skip Viragh Foundation, the Michael Rolphe Foundation, the National Institutes of Health, The International Human Frontier Science Program Organization and the Uehara Memorial Foundation.
Participating Centres
- Cancer Genome Project, Wellcome Trust Sanger Institute, Hinxton, Cambridge, UK
- Department of Haematology, University of Cambridge, Cambridge, UK
- Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins Medical Institutions, Baltimore, Maryland
- Institute for Cancer Research, Sutton, Surrey, UK
- Departments of Pathology and Oncology, Johns Hopkins
Publications:
Selected websites
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute, which receives the majority of its funding from the Wellcome Trust, was founded in 1992. The Institute is responsible for the completion of the sequence of approximately one-third of the human genome as well as genomes of model organisms and more than 90 pathogen genomes. In October 2006, new funding was awarded by the Wellcome Trust to exploit the wealth of genome data now available to answer important questions about health and disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.


