We are all mutants
An international team of 16 scientists today reports the first direct measurement of the general rate of genetic mutation at individual DNA letters in humans. The team sequenced the same piece of DNA – 10,000,000 or so letters or ‘nucleotides’ from the Y chromosome – from two men separated by 13 generations, and counted the number of differences. Among all these nucleotides, they found only four mutations.
In 1935 one of the founders of modern genetics, J. B. S. Haldane, studied men in London with the blood disease haemophilia and estimated that there would be one in 50,000 incidence of mutations causing haemophilia in the gene affected – the equivalent of a mutation rate of perhaps one in 25 million nucleotides across the genome. Others have measured rates at a few further specific genes or compared DNA from humans and chimpanzees to produce general estimates of the mutation rate expressed more directly in nucleotides of DNA.
Remarkably, the new research, published today in Current Biology, shows that these early estimates were spot on – in total, we all carry 100-200 new mutations in our DNA. This is equivalent to one mutation in each 15 to 30 million nucleotides. Fortunately, most of these are harmless and have no apparent effect on our health or appearance.
“The amount of data we generated would have been unimaginable just a few years ago. But finding this tiny number of mutations was more difficult than finding an ant’s egg in the emperor’s rice store.”
Yali Xue from the Wellcome Trust Sanger Institute and one of the project’s leaders
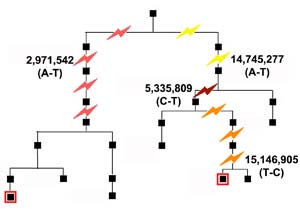
Team member Qiuju Wang recruited a family from China who had lived in the same village for centuries. The team studied two distant male-line relatives – separated by thirteen generations – whose common ancestor lived two hundred years ago.
To establish the rate of mutation The team examined an area of the Y chromosome. The Y chromosome is unique in that, apart from rare mutations, it is passed unchanged from father to son; so mutations accumulate slowly over the generations.
Despite many generations of separation, researchers found only 12 differences among all the DNA letters examined. The two Y chromosomes were still identical at 10,149,073 of the 10,149,085 letters examined. Of the 12 differences, eight had arisen in the cell lines used for the work. Only four were true mutations that had occurred naturally through the generations.
We have known for a long time that mutations occur occasionally in each of us, but have had to guess exactly how often. Now, thanks to advances in the technology for reading DNA, this new research has been possible.
Understanding mutation rates is key to many aspects of human evolution and medical research: mutation is the ultimate source of all our genetic variation and provides a molecular clock for measuring evolutionary timescales. Mutations can also lead directly to diseases like cancer. With better measurements of mutation rates, we could improve the calibration of the evolutionary clock, or test ways to reduce mutations, for example.
Even with the latest DNA sequencing technology, the researchers had to design a special strategy to search for the vanishingly rare mutations. They used next-generation sequencing to establish the order of letters on the two Y chromosomes and then compared these to the Y chromosome reference sequence.
Having identified 23 candidate SNPs – or single-letter changes in the DNA – they amplified the regions containing these candidates and checked the sequences using the standard Sanger method. A total of four naturally occurring mutations were confirmed. Knowing this number of mutations, the length of the area that they had searched and the number of generations separating the individuals, the team were able to calculate the rate of mutation.
“These four mutations gave us the exact mutation rate – one in 30 million nucleotides each generation – that we had expected. This was reassuring because the methods we used – harnessing next-generation sequencing technology – had not previously been tested for this kind of research. New mutations are responsible for an array of genetic diseases. The ability to reliably measure rates of DNA mutation means we can begin to ask how mutation rates vary between different regions of the genome and perhaps also between different individuals.”
Chris Tyler-Smith, study coordinator, also from The Wellcome Trust Sanger Institute
More information
Funding
This work was supported by the Joint Project from the NSFC and The Royal Society, and the Wellcome Trust.
Participating Centres
- The Wellcome Trust Sanger Institute, Hinxton, Cambridgeshire, UK
- Department of Otorhinolaryngology-Head and Neck Surgery, and Institute of Otolaryngology, Chinese People’s Liberation Army General Hospital, Beijing, China
- Beijing Genomics Institute at Shenzhen, Shenzhen, China
Publications:
Selected websites
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute, which receives the majority of its funding from the Wellcome Trust, was founded in 1992. The Institute is responsible for the completion of the sequence of approximately one-third of the human genome as well as genomes of model organisms and more than 90 pathogen genomes. In October 2006, new funding was awarded by the Wellcome Trust to exploit the wealth of genome data now available to answer important questions about health and disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.


