Scientists identify gene for deadly inherited lung disease
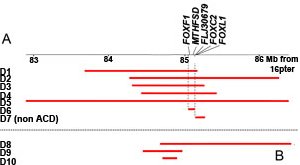
A collaboration between researchers across the globe has uncovered the genetic cause of a rare, deadly developmental disorder of the lung called alveolar capillary dysplasia with misalignment of pulmonary veins (ACD/MPV) that is usually lethal to infants within the first month of life. The disorder results from mutations in the FOXF1 transcription factor gene.
Alveolar capillary dysplasia (ACD) is a rare disorder with fewer than 200 cases reported worldwide and, until now, the underlying cause has remained elusive. This study sought to test DNA and tissue from infants born with the disorder and their parents to find the genetic causes and potentially develop a new diagnostic test for the disease.
Infants born with the disease have defects in the normal air-blood diffusion barrier in their lungs. They usually become critically ill soon after birth and they respond poorly to treatments usually used to help children who have lung or breathing problems at birth. Most die soon after birth.
“There is no question that these data are convincing. This is the gene for 30 to 40 per cent of cases. It is involved in angiogenesis – formation of new blood vessel – and lung development.”
Dr. Pawel Stankiewicz Assistant professor of molecular and human genetics at Baylor College of Medicine and first author on the paper
Because of the rarity of the disorder, the collection – with informed consent – of unique samples from 25 families whose children were born with the disease took more than a decade.
The researchers looked at slides of the lung tissue for changes of tissue structure which are a feature of ACD/MPV. The researchers then used a combination of array-based and sequencing technologies to look for the genetic changes underlying these. They found deletions that were centred around the FOX transcription factor gene cluster. After further study, the researchers found that mutations in one particular gene – FOXF1 – led to respiratory insufficiency.
“A striking feature of ACD is that infants with it often have other, additional problems at birth, most notably malformations of the heart, gastrointestinal tract and kidney. We were able to make the link between ACD and FOXF1 through our ongoing study of patients with a foregut malformation called oesophageal atresia.
“The number of different malformations that we see in patients with deletions and mutations at this locus is amazing and highlights the fundamental role that FOXF1 and its neighbours must play in early development.”
Dr Charles Shaw-Smith From the Wellcome Trust Sanger Institute and UCL Institute of Child Health and senior author on the paper
Previous studies had found that mice with deletions harbouring the equivalent Foxf1 gene exhibited abnormal alveolar development similar to that suffered in humans. These findings go further to substantiate today’s results.
The FOX transcription factor is a group of proteins involved in the regulation of the expression of genes involved in cell growth and proliferation. The genes are known to be important for early development in the womb.
The researchers also found that two patients with ACD/MPV had deletions of genetic material upstream from the FOX transcription factor gene cluster. This suggests that these regions contain elements responsible for regulation of genes in the FOX transcription factor cluster. Further studies will be needed to identify in more detail the functional role that these areas of the genome play in the development of ACD/MPV.The discovery of the function of the FOXF1 gene should improve the diagnosis in children born with the disorder and help to develop genetic counselling for families concerned about the risk of children being affected by the disease in the future.
More information
Funding
A full list of funding agencies is available at the American Journal of Human Genetics website.
Participating Centres
- Department of Molecular & Human Genetics, Baylor College of Medicine, Houston, Texas
- Department of Medical Genetics, Institute of Mother and Child, Warsaw, Poland
- Department of Pediatrics – Nutrition, Baylor College of Medicine, Houston, Texas
- Institute of Child Health, London, UK
- Wellcome Trust Sanger Institute, Hinxton, Cambridge, UK
- Signature Genomic Laboratories, LLC, Spokane, Washington
- Division of Pediatric Genetics and Metabolism, University of Florida College of Medicine, Gainesville, Florida
- Department of Pathology, Miami Children’s Hospital, Miami, Florida
- Division of Human Genetics, Children’s Hospital of Philadelphia, Pennsylvania
- Department of Pathology and Molecular Medicine, McMaster University, Hamilton, Ontario, Canada
- Department of Medical Genetics, University of Washington, Seattle, Washington;
- Regional Cytogenetics Laboratory, Addenbrooke’s Hospital, Cambridge, UK
- Department of Medical Genetics, Addenbrooke’s Hospital, Cambridge, UK
- Institute of Child Health, University College London, London, UK
- Online Mendelian Inheritance in Man
- Baylor College of Medicine (BCM)
Publications:
Selected websites
Baylor College of Medicine (BCM)
Baylor College of Medicine (BCM),Houston, Texas is the only private medical school in the greater southwest and is recognized as a premiere academic health science center known for excellence in education, research and patient care. For 2009, U.S. News and World Report ranked BCM 13th overall among the nation’s top medical schools for research and 7th for primary care. BCM is also listed 13th among all U.S. medical schools for National Institutes of Health funding, and 2nd in the nation in federal funding for research and development in the biological sciences at universities and college by the National Science Foundation. During the reaccreditation proves in March 2007, BCM received “Accreditation with Commendation” for exemplary performance in fulfilling the accreditation requirements as a provider of continuing medical education.
The UCL Institute of Child Health
The UCL Institute of Child Health is, with Great Ormond Street Hospital for Children, the largest centre for research into childhood illness outside the Americas and plays a key role in training child experts for the future. Child Health will be a key theme in the new strategic research alliance UCL Partners, recently accredited as an Academic Health Science Centre.
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute, which receives the majority of its funding from the Wellcome Trust, was founded in 1992. The Institute is responsible for the completion of the sequence of approximately one-third of the human genome as well as genomes of model organisms and more than 90 pathogen genomes. In October 2006, new funding was awarded by the Wellcome Trust to exploit the wealth of genome data now available to answer important questions about health and disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.


