Giving worms a taste of their own medicine
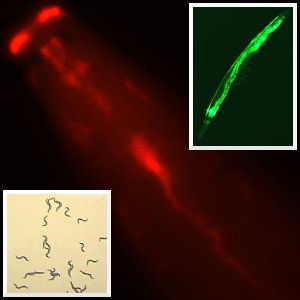 The humble nematode worm could prove invaluable in screening new compounds for active drugs, new research published today suggests.
The humble nematode worm could prove invaluable in screening new compounds for active drugs, new research published today suggests.
Soil-dwelling nematodes have a programmed avoidance response to harmful chemicals, which they detect through nerves exposed to their environment. Scientists led by the Wellcome Trust Sanger Institute have genetically modified the worm C. elegans to make human proteins called receptors in these nerves: the modified worms detect and avoid human signalling molecules and drug candidates.
The exciting results, reported today, 20 July 2006, in the open-access journal BMC Biology, promise a simple assay that can be used to screen thousands of compounds for activity against human proteins – a foundation of drug development.
“The worm is a great tool to understand biology. Because we understand it so well it has a simple well studied nervous system the role for each nerve has been mapped in detail. We also have a good understanding of the signalling mechanisms in nerves that drive the responses.
“We showed that the biochemical response of the receptors emulated that seen in humans. It is just that, in the worm, the effects of that response are to make them crawl away from the chemical stimulus. This simple response could be used to test many unknown drug candidates.”
Dr Michelle Teng of the Wellcome Trust Sanger Institute a lead author on the report
Medicines often interact with receptors, which are ‘sensors’ at the surface of cells. The team introduced the somatostatin receptor (Sstr2) and the chemokine receptor 5 (CCR5) in the nerves that respond to environmental cues. Somatostatin is a hormone that mediates a wide range of activities in humans and chemokines play an important role in the immune system. The CCR5 receptor used is also the gateway that HIV/AIDS virus uses to enter cells. Both receptors belong to a receptor family called GPCRs, which represent up to 50 per cent of current drug targets.
The response was specific. In tests, worms responded by avoiding somatostatin or chemokine placed in their paths only when the appropriate receptor was made in the appropriate nerves.
“We have shown that we can hijack the cellular machinery of the worm so that the human receptor proteins drive the avoidance response. We chose two receptors with widely differing functions in humans. The responses were specific to the compounds we added and could be inhibited in the same way a response in humans could be inhibited.”
Dr John McCafferty Principal Investigator at the Wellcome Trust Sanger Institute and senior author
The worms could also be desensitized by pre-exposure to somatostatin or chemokine: desensitization is an important part of normal human response, because it ensures that our receptors can recover for a fresh round of stimulus. This is the first time that activation has been programmed in these nerves and the team have shown that the human receptors integrate into the worm signalling machinery.
“Systems exist already to study the response of cells in test-tubes to added compounds. However, because these are soil-dwelling worms which feed on bacteria, we could test crude samples for drug candidates.”
Dr John McCafferty Sanger Institute
Together, these results make us very optimistic that these models will be widely applicable and that development of a high-throughput system is feasible.
The team used a rapid sorting system to isolate the genetically modified worms. Although for this study, worm responses were scored under the microscope, automation could be integrated to achieve a higher rate of testing.
The worm model can also help to define which regions of a novel compound are important for its biological effect, which can be crucial for producing effective drugs. The team were able to use the worm assay to identify four important building blocks within somatostatin which are known to be necessary for its effect.
“These results show the power of simple organisms such as the worm to help us not only in our understanding of biology but also in the search for new ways to improve healthcare. It is a nice irony of history that the worm was chosen for biomedical research by Sydney Brenner forty years ago in Cambridge, only a few miles from the Sanger Institute. Then twenty years ago John Sulston started to make a gene map of the animal, and eventually read its sequence as the first of all animal genomes.
“And now a new generation of researchers again in the Cambridge area uses it to test candidate drugs that are immediately relevant to human health.”
Professor Ronald Plasterk Professor of Developmental Genetics at the University of Utrecht and Director of the Hubrecht Laboratory, in the Netherlands
More information
Participating Centres and Websites
- The publication, which is available free of charge, according to BioMed Central’s open-access policy, is at: http://www.biomedcentral.com/1741-7007/4/22/
- Erasmus Medical Centre – Jansen lab – https://www.erasmusmc.nl/en/research/researchers/jansen-gert
- Publication (doi:10.1186/1741-7007-4-22)
Publications:
Selected websites
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute, which receives the majority of its funding from the Wellcome Trust, was founded in 1992. The Institute is responsible for the completion of the sequence of approximately one-third of the human genome as well as genomes of model organisms and more than 90 pathogen genomes. In October 2006, new funding was awarded by the Wellcome Trust to exploit the wealth of genome data now available to answer important questions about health and disease.
The Wellcome Trust and Its Founder
The Wellcome Trust is the most diverse biomedical research charity in the world, spending about £450 million every year both in the UK and internationally to support and promote research that will improve the health of humans and animals. The Trust was established under the will of Sir Henry Wellcome, and is funded from a private endowment, which is managed with long-term stability and growth in mind.


