Outsmarting the Smartie Bug
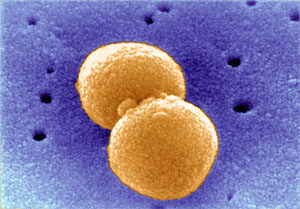 New tools in the fight against pneumococci – the bugs targeted by vaccines recently announced by the Department of Health – are described by a team led by scientists from the Wellcome Trust Sanger Institute. They have put together a complete description of the targets of the vaccine that will help monitor the disease and provide new tools for rapid diagnosis.
New tools in the fight against pneumococci – the bugs targeted by vaccines recently announced by the Department of Health – are described by a team led by scientists from the Wellcome Trust Sanger Institute. They have put together a complete description of the targets of the vaccine that will help monitor the disease and provide new tools for rapid diagnosis.
Pneumococci (formal name Streptococcus pneumoniae) are widespread, causing non-invasive disease, such as ear and sinus infections, and rarer, invasive disease, such as pneumonia and meningitis. About one in three children each year has an ear infection, of which about one-third will be due to the pneumococcus. More significantly, invasive disease is a major cause of death: around one million people, mostly young children in developing countries, die each year.
The research, published online today in PLoS Genetics, shows how the target of the vaccines, called the polysaccharide capsule, has evolved and allows the researchers to determine functions of the genes involved. The polysaccharide capsule forms a sugary coat around the bacterium and changing the structure of the capsule can help it to fool our immune defence systems – like a Smartie changing its colour.
“The bug has a polysaccharide coat which can take any one of 90 different forms, known as serotypes. The coat is essential for its ability to cause disease and its interaction with our immune system and some serotypes are more likely to be associated with disease.
“The current vaccines provide excellent protection against pneumococcal disease, but only that caused by some of the 90 serotypes, and it is important that we keep a watch on the development of this organism. Our work in describing all known variants will help in that surveillance.”
Stephen Bentley Leader of the project at the Wellcome Trust Sanger Institute
Two vaccines are available. The first, PCV, recognizes seven forms of the capsule and protects against 82 per cent of infections in children under five years of age in the UK. This will soon be given to UK children under the age of two. A second, PPV, recognizes 23 capsule types and protects against 96 per cent of the UK’s strains but is not effective in infants and is mainly used to protect the elderly.
S. pneumoniae usually lives harmlessly in the air passages and in the first year of life most people are likely to have ‘carried’ at least one strain. It is passed from person to person by sneezing and other aerosols. However, it occasionally passes from the airways to invade other tissues. This can lead to any one of a range of diseases including meningitis and infections of the sinuses, ear, lungs and blood. Why it switches to become invasive is not known.
The team sequenced all genes required to make all 90 forms of the capsule (more than 1,800,000 letters of genetic code), determined their function and studied their evolution. The work gives the most complete understanding of capsule production in any bacterial species.
The new vaccine will protect children from many of the most common serotypes but monitoring is needed to check whether other serotypes start to cause some disease. It is known that pneumococci can switch their capsular polysaccharide and so, if an invasive strain changes its coat to a form not recognised by the vaccine, it might start to become more prevalent and cause disease.
The catalogue of capsules from all known strains will help in the development of new techniques for monitoring changes in capsule type so researchers can look out for such capsule switching.
“The new vaccine that will be given to UK children is very effective at protecting against serious pneumococcal disease, but it does not protect against disease caused by the rarer serotypes. The catalogue of capsular genes will help us develop better methods to monitor the effect of the vaccine and allow us to see if changes of capsular types or increased prevalence of the rarer serotypes result in any increase in disease by serotypes not included in the vaccine.
“We must always be vigilant to changes in the properties of microorganisms when we introduce new vaccines or antibiotics. This catalogue will help us to develop new tools to keep a check on the march of the pneumococcus and is also going to give us fascinating insights into the evolution of the amazing diversity of capsular genes that can be produced by this pathogen.”
Brian Spratt Professor of Microbiology at Imperial College and a co-author of the study
Researchers are at pains to point out that current vaccines are enormously successful and vaccination plays an essential in protecting all of us. The pneumococcal vaccines have been shown to be safe and very effective at preventing widespread infections and are expected to greatly reduce serious pneumococcal disease in UK children, as they already have done in the USA.
Current research, such as the capsule study described here, is intended to ensure we keep ahead of organisms such as S. pneumoniae.
More information
Cases/incidence of pneumococcal disease (World Health Organization)
It is estimated that about 700,000 to 1,000,000 children die of pneumococcal disease every year, most of whom are young children in developing countries. In the developed world, children below two years of age and elderly persons carry the major disease burden. Growing resistance of S. pneumoniae to essential antibiotics underlines the urgent need for vaccines to control pneumococcal disease. [From: https://www.who.int/immunization/policy/position_papers/pneumococcus/en/]
In Europe and the United States, pneumococcal pneumonia is the most common community-acquired bacterial pneumonia, estimated to affect approximately 100 per 100,000 adults each year. Even in economically developed regions, invasive pneumococcal disease carries high mortality; for adults with pneumococcal pneumonia the mortality rate averages 10 per cent -20 per cent, whilst it may exceed 50 per cent in the high-risk groups. [From: https://www.who.int/ith/vaccines/pneumococcal/en/]
Participating Centres
- Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, United Kingdom
- Department of Infectious Disease Epidemiology, Imperial College, London, United Kingdom
- School of Molecular and Microbial Biosciences, University of Sydney, Sydney, Australia
- Staten Serum Institut, Copenhagen, Denmark
Funding Agencies
The work was supported a grant from the World Health Organization, and funding from the Wellcome Trust.
Publications:
Selected websites
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute, which receives the majority of its funding from the Wellcome Trust, was founded in 1992. The Institute is responsible for the completion of the sequence of approximately one-third of the human genome as well as genomes of model organisms and more than 90 pathogen genomes. In October 2006, new funding was awarded by the Wellcome Trust to exploit the wealth of genome data now available to answer important questions about health and disease.
The Wellcome Trust and Its Founder
The Wellcome Trust is the most diverse biomedical research charity in the world, spending about £450 million every year both in the UK and internationally to support and promote research that will improve the health of humans and animals. The Trust was established under the will of Sir Henry Wellcome, and is funded from a private endowment, which is managed with long-term stability and growth in mind.


