Cattle Parasite Code Cracked
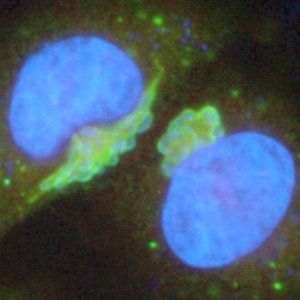
For some economies, livestock are the currency: they may be the only lifeline for health and survival. If your livestock fail, your family suffers. Treating and preventing illness in livestock is a key to success for all, but especially developing countries in Africa.
On, Friday 1 July 2005, in Science, two teams publish the genomes of parasites that threaten more than 250 million cattle, leading to major economic burdens in Africa, across the Mediterranean region, through the Middle East into India and China. Theileria annulata, which causes tropical theileriosis, is described by a team of scientists, led by researchers at the Wellcome Trust Sanger Institute.
Uniquely, Theileria, which is transmitted by ticks taking a blood meal, invades and modifies white blood cells of the unfortunate host.
“The pathogens do not simply attack their host – they interact with it and control the cells they infect. T. annulata has developed a remarkable series of strategies that allow it to invade white blood cells and direct their division. The resulting disease, in fact, resembles leukaemia with a massive destruction of white blood cells in lethal cases.”
“Without the genome sequence, it has been extremely difficult to identify the genes and the mechanisms used by the parasite to take over the host.”
Dr Matt Berriman Project manager at the Wellcome Trust Sanger Institute
Theileria is unique in its ability to ‘transform’ the cells of another animal and identifying factors involved in this process was a major goal of the sequencing project.
At 8.4 million bases, the compact T. annulata genome is about one-third the size of the Plasmodium genome, but contains more than two-thirds the number of genes. The genes are packed in so tightly that they can be hard to distinguish from one another.
“We have identified genes that may take part in hijacking the signalling machinery of the cow’s white blood cells, bringing them under the parasite’s control.”
“Several candidates in the parasite’s genetic armoury have been identified that it may use to trigger and maintain a tumour-like state of an infected host cell.”
Dr Arnab Pain at the Wellcome Trust Sanger Institute Primary study author
Also reported is the sequence of the related parasite Theileria parva, which causes East Coast Fever. The genome of the T. parva has been sequenced by a team led by researchers at the Institute for Genomic Research (TIGR) in Maryland. In addition to describing the genomic make-up of T. annulata, the WTSI team compared the genomes of these two closely related species together with other related parasites, such as malaria. Although the life-cycles of both Theileria species are similar, they are transmitted by different tick species and choose to invade different types of white blood cells and they differ in disease pathology.
Compared to the malaria parasite, the metabolism of Theileria has been reduced by evolution. In particular, the parasite’s ability to make fats and the important iron-containing molecule haem has been lost. Theileria makes up for this by scavenging key ingredients from its host. Nutrients can be taken into the parasite using transporter molecules and scientists discovered that in Theileria a large number of transporter genes flank the groups of species-specific genes at the chromosome ends.
T. annulata is highly pathogenic in European breeds of cattle resulting in up to 70 per cent mortality, and dramatically reduces productivity of indigenous breeds. Many of the current treatments focus on killing ticks using acaricides and available therapies are mostly reliable only in the early stages of the disease. Existing vaccines require improvement and resistance to the only available drug (Buparvaquone) has recently been reported.
The need for improved control and therapeutics is urgent.
“The sequence will be a major resource for all scientists working on this pathogen presenting both a challenge and an opportunity in the fields of vaccines and diagnostics. Ultimately, the genome resource will enable discovery of new therapeutics and aid vaccine development.”
Professor Andy Tait At the Veterinary School, University of Glasgow
“A major goal of several group’s research is to understand how Theileria and related parasites manipulate their mammalian host cells and cause disease, the genome resource will significantly advance these studies.”
Professor Brian Shiels At the Veterinary School, University of Glasgow
Professors Tait and Shiels are members of a consortium recently awarded £2M by the Wellcome Trust to develop sustainable methods to control tropical theileriosis, as part of “Animal Health in the Developing World”.
Tropical theileriosis and East Coast fever affect the livelihoods of the poorest farmers in developing countries, making it difficult for them to feed their families. Combating diseases such as Theileriosis is critical to allow development and battle against poverty.
More information
Publications:
Selected websites
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute, which receives the majority of its funding from the Wellcome Trust, was founded in 1992. The Institute is responsible for the completion of the sequence of approximately one-third of the human genome as well as genomes of model organisms and more than 90 pathogen genomes. In October 2006, new funding was awarded by the Wellcome Trust to exploit the wealth of genome data now available to answer important questions about health and disease.
The Wellcome Trust and Its Founder
The Wellcome Trust is the most diverse biomedical research charity in the world, spending about £450 million every year both in the UK and internationally to support and promote research that will improve the health of humans and animals. The Trust was established under the will of Sir Henry Wellcome, and is funded from a private endowment, which is managed with long-term stability and growth in mind.


