DNA of the tissue destroyer
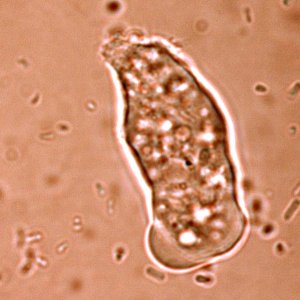
A striking new picture emerges of an organism adapting itself to life as a parasite of the human gut. It seems to have jettisoned many genes required for independent survival, relying instead on us, its human host, to help it out. At the same time, it appears to have picked up genes from bacteria in its environment – genes that allow it to use a wider range of sugars and other energy sources.
“The results give a fascinating glimpse of how this ancient parasite evolved and highlights unusual metabolic processes that may be exploitable as drug targets. The genome is full of enzymes and transporters that could be followed up by experimentation.”
Dr Matt Berriman Project Leader at the Wellcome Trust Sanger Institute
E. histolytica is a remarkable organism. Its name derives from the fact that it “eats” tissues (histo-tissue; lysis-loosen): it also has a voracious appetite for bacteria, which is how it can be grown in test tubes. Amoebic dysentery is caught by ingesting cysts – dormant organisms – from water, food or hands contaminated with human faeces. Inside the gut of human host, the parasite hatches and begins multiplying.
The sequencing of E. histolytica was a collaborative effort led by the Wellcome Trust Sanger Institute and The Institute for Genomic Research (TIGR) in Rockville, MD, USA. The project was supported by grants from the Wellcome Trust and from the National Institute of Allergy and Infectious Diseases (NIAID), which is part of the National Institutes of Health.
The sequence reveals almost 10,000 genes, some of which appear to have been acquired from bacteria. Nearly one-third of the genes are unrelated to genes in public databases.
“Entamoeba histolytica is a major cause of acute diarrhoea in developing countries, but the research community working to combat it is relatively small. The draft sequence provides a much-needed boost, and our continuing efforts to improve our understanding will strengthen these efforts.”
Dr Matt Berriman Wellcome Trust Sanger Institute
A defining feature of E. histolytica is its voracious appetite – it lives in an environment where it is surrounded by and eats bacteria. In the human gut, it causes diarrhoea, fluid and salt loss and bleeding from the gut. It can also invade the gut wall and spread to other tissues, causing more serious disease.
“Clearly, this amoeba has genes that allow it to sense certain facets of its environment and respond to those cues.”
Neil Hall A Sanger Institute scientist, now at TIGR, who is the senior author of the Nature study
It was long considered to be a “primitive” organism – originating from the time that bacteria and more complex organisms diverged in evolution, because it lacks many of the characteristic components of more complex cells. It also has some features typical of bacteria.
One emerging and strengthening picture from the sequence of parasite genomes is the number of genes that successful organisms use to evade our defence mechanisms. E. histolytica has large “families” of genes used to produce its surface coat. It is thought that it may use different coats to prevent detection in the human body for years at a time.
The range of genetic tricks surprised the research team, but also leads to hope.
“It is also unusual for so many bacterial-like genes to be used in this way. Taken together these findings open up a lot of possibilities for producing selective inhibitors – drugs that affect the organism but don’t affect us.”
Dr Matt Berriman Sanger Institute
More information
-
The paper is published by Nature: Loftus B et al. (2005) The genome of the protist parasite Entamoeba histolytica.
-
The genome sequence consists of 23,751,783 base-pairs (bp). The 9938 predicted genes average 1170 bp in size and comprise 49% of the genome. No identifiable homologues could be identified for one third of predicted proteins (31.8 per cent) from the public databases
-
For 96 genes the authors conclude that the current evidence is most consistent with gene transfer from prokaryotes to the E. histolytica genome.
-
There are an estimated 50 million cases worldwide of amoebiasis with up to 100,000 deaths annually, making it the second-biggest killer after malaria for a protozoan parasite. About one in ten people infected become ill. (Source)
Participating Centres
The Institute for Genomic Research (TIGR) is a not-for-profit research institute based in Rockville, Maryland. TIGR, which sequenced the first complete genome of a free-living organism, has been at the forefront of the genomic revolution since the institute was founded in 1992. The Institute’s scientists conduct research involving the structural, functional, and comparative analysis of genomes and gene products in viruses, bacteria, archaea, and eukaryotes.
-
The Wellcome Trust Sanger Institute, The Wellcome Trust Genome Campus, Hinxton, Cambridge CB10 1SA, UK
-
TIGR, 9712 Medical Center Drive, Rockville, Maryland 20850, USA
-
School of Biology, University of Newcastle, King George VI Building, Newcastle upon Tyne NE1 7RU, UK
-
Department of Molecular and Cell Biology, Boston University Goldman School of Dental Medicine, 715 Albany Street, Boston, Massachusetts 02118, USA
-
Departments of Internal Medicine & Microbiology, University of Virginia, Charlottesville, Virginia 22908, USA
-
Department of Parasitology, National Institute of Infectious Diseases, 1-23-1 Toyama, Shinjuku-ku, Tokyo 162-8640, Japan
-
Division of Specific Prophylaxis and Tropical Medicine, Center for Physiology and Pathophysiology, Medical University of Vienna, Kinderspitalgasse 15, A-1095 Vienna, Austria
-
Department of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT, UK
-
Department of Molecular Parasitology, Bernhard Nocht Institute for Tropical Medicine, Bernhard Nocht Str. 74, 20359 Hamburg, Germany
-
Zoological Institute, University of Kiel, Olshausenstr. 40, 24098 Kiel, Germany
-
School of Environmental Sciences, Jawaharlal Nehru University, New Delhi 110067, India
-
Unite de Biologie Cellulaire du Parasitisme, INSERM U389, Institut Pasteur 28, rue du Dr Roux 75724, Paris Cedex 15, France
-
Department of Biochemistry, Bose Institute, P1/12 CIT Scheme VIIM, Kolkata 700054, India
-
Department of Zoology, The Natural History Museum, Cromwell Road, London SW7 5BD, UK
-
Center for Biological Sequence Analysis, Technical University of Denmark, Building 208, DK-2800 Lyngby, Denmark
-
Departments of Internal Medicine, Microbiology, and Immunology, Stanford University School of Medicine, Stanford, California 94305-5107, USA
Publications:
Selected websites
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute, which receives the majority of its funding from the Wellcome Trust, was founded in 1992. The Institute is responsible for the completion of the sequence of approximately one-third of the human genome as well as genomes of model organisms and more than 90 pathogen genomes. In October 2006, new funding was awarded by the Wellcome Trust to exploit the wealth of genome data now available to answer important questions about health and disease.
The Wellcome Trust and Its Founder
The Wellcome Trust is the most diverse biomedical research charity in the world, spending about £450 million every year both in the UK and internationally to support and promote research that will improve the health of humans and animals. The Trust was established under the will of Sir Henry Wellcome, and is funded from a private endowment, which is managed with long-term stability and growth in mind.


